Article Text
Abstract
Objectives To examine concentrations of perfluoroalkyl substances (PFASs) and lifestyle factors that may contribute to higher levels of pollutants in never-pregnant women of fertile age.
Design Observational cross-sectional study.
Setting Participants were recruited among employees and students at Haukeland University Hospital and the University of Bergen, Norway.
Participants Healthy, never-pregnant Norwegian women (n=158) of fertile age (18–39 years).
Outcomes Concentrations of 20 different PFASs, mercury (Hg), lead, cadmium, total, high-density lipoprotein and low-density lipoprotein (LDL) cholesterol, in addition to self-reported data on dietary intake.
Results Seven PFASs were detected in more than 95% of the women. Women aged 30–39 years had higher concentrations of sum PFAS compared with younger women. Serum PFASs were significantly intercorrelated (rho: 0.34–0.98, p<0.001) and six of them were significantly correlated to whole blood Hg (rho: 0.21–0.74, p<0.01). Fish consumption was the strongest predictor for most serum PFASs and for whole blood Hg. Fish consumption and serum perfluorooctanesulfonic acid (PFOS) concentrations were both positively associated with serum total and LDL cholesterol, established risk factors for cardiovascular disease.
Conclusions The majority of Norwegian never-pregnant women of fertile age had a mixture of seven different PFASs and Hg detected in their blood. PFAS concentrations were higher in older women and associated with fish intake. As the mean age of women at first birth is increasing, several factors require further consideration including diet, as this may influence the burden of PFAS to the next generation.
Trial registration number ClinicalTrials.gov ID: NCT03272022, Unique Protocol ID: 2011/2447, Regional Committee for Medical Research Ethics West (2011/2447), 12 January 2012.
- dietary patterns
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What this paper adds
In non-pregnant women aged 18–39 years, older women (30–39 years) had higher concentrations of sum PFAS as compared to young women.
The most common choice for fish dinner was farmed salmon, and fish consumption was the strongest predictor for most serum PFASs.
Fish consumption and serum PFOS concentrations were both positively associated with serum total and LDL cholesterol, which established risk factors for caridovascular disease.
Background
Perfluoroalkyl substances (PFASs) are a group of synthetic carbon fluorine compounds that have been widely used in various consumer products for more than 50 years due to their water-repelling and stain-repelling features.1 All people in industrialised countries have measureable amounts of PFASs in their blood1 and even in remote areas, selected PFASs are detectable in humans.2 3
Parity is a strong predictor of PFAS concentrations. Women are reported to have lower PFAS concentrations with increasing parity, implying that the PFAS load is higher in the first-born child.4 PFASs pass the placenta and accumulate in fetal blood and tissues during pregnancy.5 PFASs have endocrine disrupting properties and negative effect on the immune system and neurodevelopment.1 In adults, dyslipidaemia, with elevated total cholesterol and low-density lipoprotein (LDL) cholesterol, or reduced high-density lipoprotein (HDL) cholesterol, is considered the strongest metabolic effect of PFAS exposure.1
Many PFASs are environmentally persistent and accumulate in the food chain.1 6 The European Food Safety Authority (EFSA) has recently estimated that fish and other seafood account for up to 86% of dietary PFAS exposure in adults.7 Fish is an important source of nutrients; however, due to environmental contamination, fish intake is associated with a mixture of pollutants,8 including dioxins and dioxin-like polychlorinated biphenyls (PCBs),9 and mercury (Hg),10 substances with established negative health effects.1 11 12
PFAS concentrations are demonstrated to be higher in older individuals,13 and as the mean age of women at first birth is getting higher in the Western world,14 this may increase the burden of toxic substances in newborns. In order to reduce the transfer of pollutants to the next generation, it is important to know the PFAS status and the risk factors that contribute to higher concentrations in the fertile population, particularly in first time mothers.
The aim of the present study was to measure serum concentrations of 20 PFASs in healthy, never-pregnant Norwegian women of fertile age (18–39 years) and investigate factors associated with a higher burden of pollutants and effect on lipid status. We have included formerly published data on total Hg, lead (Pb) and cadmium (Cd)10 to look for correlations that might indicate common sources of exposure.
Methods
Study population and design
Between June 2012 and March 2015, 158 healthy, never-pregnant women aged 18–39 years were recruited among employees and students at Haukeland University Hospital and the University of Bergen, Norway.
Patient and public involvement
No patients were involved in the design and recruitment of the study.
Health assessment questionnaire
The women completed a simple, non-validated, questionnaire concerning age, body weight, health status, years of completed education, diet, including intake of fish for dinner, and the use of micronutrient supplements, alcohol and tobacco. The women were categorised as ‘healthy’ and eligible for inclusion if they reported to never have been pregnant and had no chronic illness except for well-regulated hypothyroidism and no regular use of medications except for thyroxin medication. Fish for dinner was categorised as never, <1 times/month, 1–3 times/month, 1–2 times/week and 3–5 times/week. Regular use of micronutrient supplements was defined as use more than 3 days per week and the definition of a regular tobacco user was based on a plasma cotinine concentration >85 nmol/L.15
Blood sampling and analysis
Non-fasting blood samples, obtained by antecubital venipuncture and collected into vacutainer tubes without additives (Terumo), were collected from morning to early afternoon in the period from 2012 to 2015. Serum was transferred into Sarstedt tubes without additives with plastic pipettes, and stored at − 80°C. For analysis of PFAS, the samples were shipped to the University Hospital of North Norway (Tromsø, Norway) and were stored at −30°C between 1 and 4 years prior to analysis in 2016. PFASs are reported to be stable in human serum even in room temperature for several months.16 The sampling equipment underwent testing for background contamination, which was not present.
Twenty different PFASs were analysed according to Huber and Brox17 by an automated fully validated high-throughput sample preparation method and analysis by ultrahigh pressure liquid chromatography triple-quadrupole mass-spectrometry (LC-MS/MS, Waters, Milford, Massachusetts, USA). Analysed PFASs consist of perfluorobutanoate (PFBA), perfluoropentanoate (PFPA), perfluorohexanoate (PFHxA), perfluoroheptanoate (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoDA), perfluorotridecanoate (PFTrDA) and perfluorotetradecanoate (PFTeDA), perfluorobutane sulfonate (PFBS), perfluoropentane sulfonate (PFPS), perfluorohexane sulfonate (PFHxS), perfluoroheptane sulfonate (PFHpS) and perfluorooctanesulfonic acid (PFOS), perfluorononane sulfonate (PFNS), perfluorodecane sulfonate (PFDS), perfluorododecane sulfonate (PFDoDS) and perfluorooctane sulfonamide (PFOSA). Linear species (lin) and sum of linear and branched species (sum) were quantified for PFHxS, PFHpS and PFOS, while sum of perfluorocarboxylic acids (PFCAs), sum of perfluorosulfonic acids (PFSA) and sum of all quantified perfluoroalkyl substances (PFAS=PFCA+PFSA) were calculated. PFBA and PFPA were not further taken into account in the present study since a specific identification was not possible without applying a complementary analysis method.17 For quality assurance, 4 blank samples, 4 SRM 1958 (NIST, Gaithersburg, Maryland, USA) and 4 SRM 1957 (NIST) samples and 3 bovine serum samples (Sigma Aldrich, Steinheim, Germany) were analysed within each batch of 96 samples to control for background and carry-over effects. All the quality controls were within the acceptance limits. Analytical coefficients of variation (CVa) were ≤10% for all the measured PFASs except for PFDA and PFUnDA with CVa of 12% and 18%.
Serum total, LDL and HDL cholesterol were analysed in the routine clinical laboratory at Haukeland University hospital, Bergen, Norway on Roche Cobas c702 (Roche Diagnostics, Basel, Switzerland) automated analyzer. CVa for total cholesterol was 3%, LDL cholesterol 2.5% and for HDL cholesterol 3%.
Plasma levels of cotinine were assayed by Bevital.no, using an LC-MS/MS method with a lower limit of detection (LOD) of 1 nmol/L.18 Formerly published data on whole blood concentrations of Hg, Pb and Cd were included.10
Statistical analysis
The results for age and body mass index (BMI) are presented as mean and SD. PFAS concentrations were not normally distributed, and are presented as median, IQR and total range. Mann-Whitney U test was used to compare between groups for continuous data and χ2 test for categorical data. Spearman correlation and multiple linear regression were used to explore relationships between PFAS concentrations and lifestyle factors. Age, BMI and fish consumption, factors known to be associated with PFAS concentrations, were entered simultaneously into the multiple linear regression models. Graphical illustrations of the relationships between various PFASs, Hg, serum total and LDL cholesterol were obtained by generalised additive models.
LODs for the PFAS analyses were set as concentrations calculated by the TargetLynx software for each individual sample (LODi) and each individual analyte with a signal to noise ratio of 3 divided by the related sample amount. Where blank contamination was detected (background contribution during sample preparation), LOD was calculated as an average of the blanks multiplied by three times of their SD. If the LOD calculated from the blank contamination was higher than the LODi of the sample, the LOD calculated based on the blank samples was used. Limit of quantification (LOQ) was defined as three times the LOD. To reduce possible bias of left censored data analyses we have used the actual values between LOQ and LOD. PFAS concentrations below the LOD were not quantified (in most cases there was only noise visible in the chromatogram) and these data were replaced by LODi divided by 2.
Statistical analyses were performed only for PFASs with detection rate >90%. PFASs with detection rate <90% were included in the PFASs sum concentration (ΣPFAS) as well as sum PFCAs and sum PFSAs.
The SPSS statistical programme (.V24) and the packages ‘mgcv’ in R, V.3.3 (The R Foundation for Statistical Computing) were used for the statistical analyses. Two-sided P values<0.05 were considered statistically significant.
Results
Demographics
The participants were healthy, young women with a mean age of 25 (SD 5), range 18–39 years and a median BMI of 21.8 (IQR 20.6, 23.7, range 17.3–35.2). More than 90% of the women had ≥12 years of education. Reported median use of alcohol was 2 units per week (IQR 0.5, 3.8), ranging from 0 to 12 units. Based on plasma cotinine levels≥85 nmol/L, 18/158 (11%) women were defined as regular tobacco users. Omega 3 fatty acids were the most commonly used supplement and used by 44%, while multivitamin supplements were used by 22% of the women. The majority had a varied diet, while 31/158 (20%) women reported that they never ate meat or fish. Overall, 4% of the women had fish for dinner <1 times/month, 22% 1–3 times/month, 43% 1–2 times/week and 12% 3–5 times/week. Farmed salmon was the most frequently used type of fish (71%), followed by lean fish (17%) and other types of fatty fish (10%).
Serum PFAS concentrations in never-pregnant women of fertile age
Median, IQR and concentration range of the seven most frequently detected PFASs, including linear and sum of linear and branched species of PFHpS, PFHxS and PFOS, and total sum of PFCAs, PFSAs and PFASs, are shown in table 1. PFOA, PFNA, PFDA, PFHxS and PFOS were detected in serum of all 158 women, while PFUnDA was detected 155 women (98%) and PFHpS in 151 women (96%). The remaining PFASs were detected in less than 70% of the samples: PFHxA (1%), PFHpA (45%), PFDoDA (13%), PFTrDA (24%) and PFTeDA (0%), PFBS (37%). PFPS, PFNS, PFDS, PFDoDS and PFOSA were all below LOD in 100% of the study population.
Serum PFAS concentrations in never-pregnant women aged 18–39 years, n=158
PFAS concentrations were strongly intercorrelated (rho: 0.34–0.98, p<0.001). Significant positive correlation to age was seen for PFUnDA (rho=0.20, p=0.01), and sum PFOS (rho=0.24, p=0.002), and weaker correlations were observed for age and sum PFSA and sum PFAS, (rho >0.17, p<0.03). Women aged 31–39 years (n=23) had up to 50% higher median concentrations of sum PFOS, sum PFSA and sum PFAS compared with younger women (table 2).
Serum PFAS concentrations in younger versus older never-pregnant women, N=158
No significant correlations were observed between any PFAS concentration and BMI or alcohol intake. Plasma cotinine concentrations were negatively correlated to sum PFOS, sum PFSA and sum PFAS (rho:<−0.16, p<0.04). Smokers had slightly lower median lin PFOS concentrations 1.73 (IQR 1.31, 3.30 ng/mL, compared with non-smokers 2.51 (IQR 1.75, 3.52 ng/mL), p=0.048. Smokers (n=18) had lower intake of fish than non-smokers (n=140), only 22% of the smokers had fish for dinner once or more per week, compared with 59% of the non-smokers, p=0.01.
Fish consumption was positively correlated to all investigated PFASs (rho: 0.18–0.50, p<0.03), except for PFOA, lin and sum PFHxS. The same pattern was seen in multiple linear regression models, which additionally included age and BMI (table 3).
Determinants of serum PFAS concentrations in never-pregnant women aged 18–39 years by multiple linear regression, n=158
Relations between PFASs and inorganic pollutants
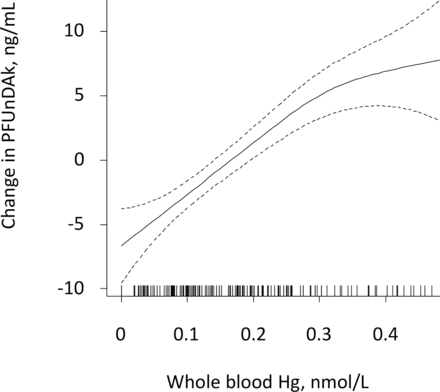
Median (IQR) whole blood Hg was 4.91 (1.70, 8.68) nmol/L, whole blood Pb 0.04 (0.03, 0.05) µmol/L and whole blood Cd 1.53 (1.15, 2.18) nmol/L, as formerly reported.10 Whole blood Hg was detected in 88% of the women and was significantly correlated to six of the most frequently detected serum PFASs: PFOA (rho: 0.21, p=0.01), PFNA (rho: 0.51, p=0.001), PFDA (rho: 0.60, p<0.001), PFUnDA (rho: 0.74, p<0.001), sum PFHpS (rho: 0.30, p<0.001), sum PFOS (rho: 0.39, p<0.001). A graphical illustration of the strongest correlation, (between whole blood Hg and PFUnDA), is shown in figure 1. Whole blood Hg was also strongly correlated to fish consumption (rho: 0.68, p<0.001). No significant correlations to individual or sum PFASs were observed for whole blood Pb or Cd.
Serum perfluoroundecanoate (PFUnDA) in relation to whole blood mercury (Hg) in never-pregnant women aged 18–39 years, by generalised additive models. The values on the y-axes are given as difference from the respective mean values.
Relations between PFASs and cholesterol
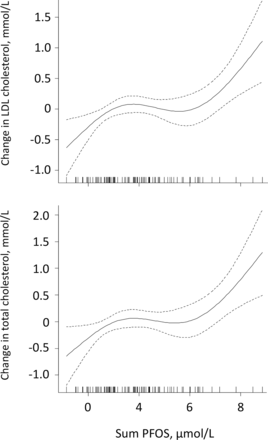
Median (IQR) serum total cholesterol concentration was 4.3 (3.8, 4.7) mmol/L, LDL cholesterol 2.4 (2.0, 2.9) mmol/L and HDL cholesterol 1.8 (1.5, 2.1) mmol/L. Significant positive correlations were seen between PFOS (lin and sum) and total and LDL cholesterol (rho >0.25, p<0.02) (figure 2). No other PFASs correlated significantly with total or LDL cholesterol, and no significant correlations were seen between individual or sum PFASs and HDL cholesterol.
Serum total and low-density lipoprotein (LDL) cholesterol in relation to serum sum PFOS in never-pregnant women aged 18–39 years, by generalised additive models. The values on the y-axes are given as difference from the respective mean values. sum PFOS, sum perfluorooctanesulfonic acid.
Intake of fish for dinner was positively correlated to total cholesterol (rho=0.25, p=0.02) and LDL cholesterol (rho=0.20, p=0.06), but not to HDL cholesterol.
PFOS (lin) were the strongest predictor for total and LDL cholesterol concentrations in multiple linear regression models, which additionally included age, BMI and fish consumption (table 4).
Determinants of serum cholesterol concentrations in never-pregnant women aged 18–39 years by multiple linear regression, n=158
Discussion
Seven different PFASs were detected in more than 95% of healthy Norwegian never-pregnant women. Women aged 30–39 years had up to 50% higher median concentrations of sum PFOS, PFSA and PFAS compared with younger women. Six of the PFASs (PFOA, PFNA, PFDA, PFUnDA, sum PFHpS and sum PFOS) were significantly correlated to whole blood Hg. Fish consumption was the strongest predictor for all PFAS concentrations, except for PFOA and PFHxS, and for whole blood Hg. Serum PFOS concentration and fish consumption were both positively associated with total and LDL cholesterol concentrations.
There are more than 4500 different PFASs, but the most studied are PFOS and PFOA.1 PFOS was restricted according to the Stockholm convention list in 2011, and PFOA was included in 2019 (https://www.sciencedirect.com/topics/earth-and-planetary-sciences/stockholm-convention). The EFSA reduced in 2018 the tolerably weekly intake (TWI) of both PFOS and PFOA;19 however, according to the EFSA, a considerable proportion of the population exceeds the proposed TWIs of both compounds.
Several studies have examined PFASs status in the non-pregnant women,20 21 including the impact on cholesterol concentrations.22 A non-pregnant population may, however, contain parous women, and as many PFASs are transferred from the mother to the fetus and infant during pregnancy and breast feeding, respectively, this will influence PFAS concentrations. In a Norwegian study, pregnant parous women had 46% lower PFOS and 70% lower PFOA than pregnant, nulliparous women.23 Few studies have examined PFAS status in never-pregnant women of fertile age. In 2014, Chinese nulliparous women, aged 20–40 years, had higher median sum PFAS (12.16 ng/mL), PFOA (5.07 ng/mL), PFNA (0.52 ng/mL) and PFDA (0.45 ng/mL), but lower PFOS (4.05 ng/mL) and PFHxS (0.24 ng/mL) concentrations, compared with our population of Norwegian women.24
The measured PFAS concentrations in the Norwegian women are more or less comparable to the levels reported in Norwegian adolescents aged 15–19 years from the Artic area.25 All PFASs, except PFOA and PFHxS, showed a positive correlation to age, particularly PFOS and PFUnDA, which have also been demonstrated in other cross-sectional studies.13 23 26 As both inorganic and organic pollutants show higher concentrations in individuals of higher age,13 older first time mothers may transfer a higher amount of pollutants to the child during pregnancy and lactation. Advanced maternal age is associated with health problems in the offspring, including hypertension, obesity, diabetes and cancer.27 The same conditions have been related to effects of PFAS1 and other persistent organic pollutants.28
Serum concentrations of the various PFASs were strongly intercorrelated, and all, but PFHxS, were highly correlated to whole blood Hg, particularly PFUnDA and Hg. The same pattern was observed in a US population,29 and most likely reflects a common source of these six PFASs and Hg. In our population, fish consumption was the strongest determinant of PFAS concentrations and strongly correlated with whole blood Hg,10 which are in accordance with other published studies.13 23 25 26 30–33
Farmed salmon was reported to be the most common choice of fish for dinner and omega 3 fatty acids were the most common supplement used by the women. These dietary habits reflect current Norwegian recommendations (https://helsenorge.no/kosthold-og-ernaring/kostrad/spis-fisk-oftere), which is to eat fish for dinner two to three times per week. Approximately half of this should be fatty fish, known to be rich in omega 3 fatty acids, which has been considered to reduce the risk of metabolic syndrome and cardiovascular disease in particular.34 A recently published 13-year follow-up study suggested, however, that lean fish, but not fatty fish consumption, seems to be associated with beneficial changes in metabolic syndrome.35 This difference may be related to the contamination profile in fatty versus lean fish. Previous studies have demonstrated an association between intake of fatty fish and fish oil and higher serum concentrations of persistent organic pollutants like PFASs23 and dioxins, and dioxin-like PCBs.9 While both organic and inorganic pollutants are associated with numerous negative health effects,1 11 12 it has been advocated that the beneficial effects of fish counteract the negative effects of these pollutants.36 However, the observed relation between PFOS and dyslipidaemia challenges this belief. High total and LDL cholesterol are established major risk factors for cardiovascular disease,37 and PFOS, found to be solely related to intake of seafood,23 is associated with higher serum total and LDL cholesterol concentration.38 The common belief that increasing your intake of marine omega 3 fatty acids by consuming more fatty fish and fish oil supplements reduces the risk of heart disease, stroke and death, have repeatedly been questioned,39 and a recent Cochrane review concluded that increasing the intake of omega 3 fatty acids has little or no effect on mortality or cardiovascular health40 and should not be recommended.41 Fish is an important nutrient, but the contamination of fish and seafood must be taken into consideration when giving dietary advice, particular for vulnerable groups, such as children and women of fertile age.42
Fish, and particularly fatty fish is an important source of vitamin D, and vitamin D deficiency is reported to be a problem when UV-B radiation is sparse.43 If fish consumption is reduced, alternative food sources of vitamin D, such as egg, cheese, fortified foods or supplements need to be included in the diet, particularly during wintertime in high latitudes.44
We did not find any significant correlations between PFASs and BMI. Apart for a significant correlation between PFHxS and BMI in Norwegian pregnant women, no correlation between PFASs and BMI have been reported in other studies.23 Higher PFOA concentrations have been reported in smokers,23 and positive associations between PFOA, PFNA and PFDA sum PFHxS and sum PFAS are reported in chewed tobacco users.25 Interestingly, smokers in our population had lower lin PFOS concentrations, a finding which have been reported before.23 45 We have formerly reported that smoking women of fertile age also have lower Hg concentrations.10 These associations may be explained by different lifestyle patterns in smokers versus non-smokers. Smokers consumed less fish and their level of PFOS and Hg, which are both related to fish intake,10 23 are, therefore, lower. Eating fish is considered to be part of a healthy lifestyle, while smoking is not. Intake of seafood has in fact, been suggested as a proxy for a healthy lifestyle,46 something which may contribute to an overestimation of the positive health effects related to fish intake.
This was a cross-sectional, observational study with a small sample size, which represent a limitation to the study. The dietary data were self-reported and the cross-sectional nature of the data does not permit conclusions about causality of the associations. Our study describes PFAS status in Norwegian never-pregnant women between June 2012 and March 2015, and is not representative for PFAS status in other populations or in other time periods. Unmeasured confounding variables and temporality issues are a problem in drawing causal inferences in observational studies. As exposure and outcome were measured at the same time, any correlation may reflect mutual dependence on some unknown, time-related variable.
Conclusion
The majority of Norwegian never-pregnant women of fertile age had a mixture of seven different PFASs and Hg detected in their blood. PFASs concentrations were higher in older women and associated with fish intake. As the mean age of women at first birth is increasing, several factors require further consideration including diet, as this may influence the burden of PFAS to the next generation.
Acknowledgments
The authors thank Christina Ripman Hansen and Sten-Kristian Odden, Department for Laboratory Medicine, University Hospital of North Norway, for their help with sample preparation and analysis of perfluoroalkyl substances. The authors acknowledge the financial support of the Department for Laboratory Medicine, University Hospital of North Norway and Northern Norway Regional Health Authority for direct strategical financial support of the Laboratory.
References
Footnotes
Contributors All authors contributed to the study conception and design. Data collection were performed by KV and A-LB-M. Analysis were performed by KV, A-LB-M and SH. The first draft of the manuscript was written by A-LB-M and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Ethical approval of the protocol was granted by the Regional Committee for Medical Research Ethics West (2011/2447). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all women. This study was performed in line with the principles of the Declaration of Helsinki.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.