Article Text
Abstract
Malnutrition in critical care is highly prevalent and well documented to have adverse implications on morbidity and mortality. During the current COVID-19 pandemic, the evolving literature has been able to identify high risk groups in whom unfavourable outcomes are more common, for example, obesity, premorbid status, male sex, members from the Black, Asian and Minority Ethnic (BAME) community and others. Nutritional status and provision precritical and pericritical phase of COVID-19 illness is gaining traction in the literature assessing how this can influence the clinical course. It is therefore of importance to understand and address the challenges present in critical care nutrition and to identify and mitigate factors contributing to malnutrition specific to this patient group. We report a case of significant disease burden and the associated cachexia and evidence of malnutrition in a young 36-year-old male with Somalian heritage with no pre-existing medical conditions but presenting with severe COVID-19 during the first wave of the pandemic (March 2020). We highlight some key nutritional challenges during the critical phase of illness signposting to some of the management instigated to counter this. These considerations are hoped to provide further insight to help continue to evolve nutritional management when treating patients with COVID-19.
- COVID-19
- malnutrition
- nutrition assessment
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
Nutritional screening and appropriate dietetic-led interventions remain the cornerstones of success in preventing and managing malnutrition in critical care. Patients undergo a multitude of physiological and metabolic changes as a result of infection, trauma, inflammation, cytokine storm and hormonal dysregulation predisposing the individual to malnutrition.1 A common sequelae of critical illness and in this particular case of COVID-19 is the role of inflammation to the respiratory tract and the latter ‘cytokine storm’ with associated catabolic effects to the individual.2
Malnutrition in critical care is not only defined by weight loss but also muscle wasting, sarcopenia and metabolic dysregulation, which have shown to worsen clinical outcomes and have longer term impacts of physical debilitation and decreased quality of life.3 Our evolving understanding of the pathophysiology of COVID-19 has sweeping nutritional implications in the critical care setting. Chiefly, the significant catabolism and increased nutritional requirements which have been observed in cases using indirect calorimetry.4 Primary COVID-19-related changes, namely pneumonitis and immune-driven inflammatory responses, and secondary factors such as ‘cytokine storm’, fibrotic damage to the lung parenchyma increasing ventilatory requirements, concurrent bacterial infection and risk of micro-emboli necessitating renal replacement therapy, all increase catabolic and micronutrient demands.
It is therefore the role of the clinical team and dietitian to help attenuate such changes via optimisation of nutritional intake and provision. Although many critical care units will have nutritional screening in place, amidst the COVID-19 pandemic blanket referrals and feeding pathways have been implemented to enhance nutritional services and minimise individuals being overlooked. This is on the backdrop of appreciating a dramatic change in working environments, chiefly the initial influx of patients and logistical issues, for example, redeployment of staff less skilled within critical care dietetics and conducting remote reviews to preserve personal protective equipment (PPE) and reduce footfall. Professional bodies have helped to advise and steer recommendations to aid this and clinical guidelines rapidly synthesised to help form a basis of nutritional management specific for patients with COVID-19 such as the British Dietetic Association (BDA) and European Society of Clinical Nutrition and Metabolism (ESPEN).5 6 It is recognised that current guidance is expert consensus drawn from near similar pathologies, for example, acute respiratory distress syndrome (ARDS) and routine nutritional critical care management and thus remains non-specific to COVID-19. However, a unifying theme addressed is the deleterious effects of malnutrition on short-term and long-term clinical outcomes and how and which nutritional strategies can be adopted to help address these.
Case presentation
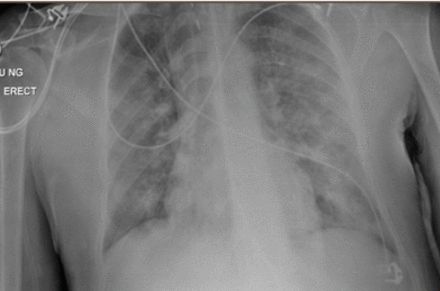
A 36-year-old man was transferred to intensive care unit (ICU) at a tertiary centre after a 24-hour period of being intubated and ventilated secondary to worsening type 1 respiratory failure. The presentation was consistent with COVID-19 with correlating symptomology describing a 1-week history of persistent fever, dry cough and 3 days of coryzal symptoms. Radiographic features included lower/mid zone and multiple peripheral opacities described as moderate to severe disease status (figure 1). This was later confirmed with a positive SARS-CoV-2 PCR swab result returning 48 hours into admission.
CXR (AP erect) indicating radiographic evidence of COVID-19 (erect anteroposterior chest view)
Baseline biochemical markers were equally in keeping with a diagnosis of COVID-19. Admission bloods indicated a proinflammatory process with a raised C-Reactive Protein (CRP) (320 mg/L), ferritin (2020 µg/L), D-dimer (2262 ng/mL) and a lymphocytopaenia (0.7×109/L) in keeping with COVID-19.7 Furthermore, a concurrent neutrophilia indicating possible superimposed bacterial infection. During this admission, nutritional specific markers were collected indicating a normal Glycated Haemoglobin (HbA1c) and lipid profile as obtained within the first week of admission. Serum vitamin D—25-hydroxyvitamin D (25(OH)D) was further measured at week six indicating insufficient levels at 34 nmol/L.
Nutritional assessment was completed post transfer to the tertiary ICU and was conducted remotely in line with local guidance, which involved restricted access to the unit preserving PPE and footfall. Initial anthropometric data were collected using estimated weight/height (75 kg/1.75 m2) providing a Body Mass Index (BMI) 24.5 kg/m2 although later corrections with the patient indicated initial body weight to be 80–85 kg (minimum of 5 kg discrepancy) giving a BMI of ~26.1 kg/m2 (overweight category). Early nasogastric tube placement enabled enteral tube feeding whereby a number of predictive energy equations were used to estimate energy and protein requirements noting the limited guidance specific to patients with COVID-19. These included the use of the Penn State protocol (Mifflin-St.Jeor formula) which equated to 1631 kcal/day and using ESPEN recommendations of 15–20 kcal/kg during the initial ebb phase of disease which provided a range of 1125–1500 kcal/day (calculated based on initial estimated body weight of 75 kg).8 The latter equation was adopted as per department recommendations to help simplify the assessment process as part of the wider contingency planning for dietetic care. Ongoing assessment aimed to ensure continual re-evaluation of nutritional requirements and was later adjusted to reflect 20–25 kcal/kg in keeping with ESPEN recommendations. Protein requirements were initially estimated between 90 and 112.5 g protein/day based on 1.2–1.5 g protein of actual body weight.
Initially large volumes of sedation and paralysis were required. The use of propofol was factored into calculations of total energy provision, which initially exceeded 300 mg/hour, equivalent to over ≥350 kcal/day in the first week. When calculating baseline requirements, sepsis was the primary factor considered noting the patient had no significant medical history, no further comorbidities and was previously well nourished and physically active. The patient was not on any regular medications or over the counter supplements prior to admission.
Treatment
Across the course of the prolonged ICU admission, the key nutritional goals were to ensure nutritional provision focused on attenuating the detrimental effects of critical illness on nutritional state, addressing increased energy deficit, catabolism and sarcopenic changes. This all on the backdrop of working in uncharted clinical territory in the form of managing critical illness caused by the COVID-19 and manifestation of COVID-19. This case in particular was complicated by the extent and severity of COVID-19 disease and the prolonged medical treatment required to manage this. This consisted of 52 days in total on the critical care unit with 50 of these requiring full ventilatory support.
Poor lung compliance alongside brittle ventilation and oxygenation resulted in a protracted ventilator and tracheostomy wean. The clinical course was further complicated halfway through admission as clinically suspected pulmonary embolism (PE) was confirmed by CT pulmonary angiogram. This identified a non-occlusive right lower lobe segmental and subsegmental PE and demonstrated that 75% of the lung parenchyma had extensive COVID-19 pneumonitis with early evidence of fibrotic change. Multiple antibiotic therapies were initiated for concurrent bacterial infection (indicated by elevated procalcitonin) alongside the adjunct of ventilating in the prone position (12 periods of proning equating to 196 hours) having metabolic and logistical impacts to nutritional provision whereby low volume feeding was adopted for these time periods.5 9
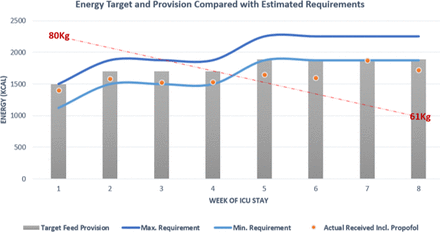
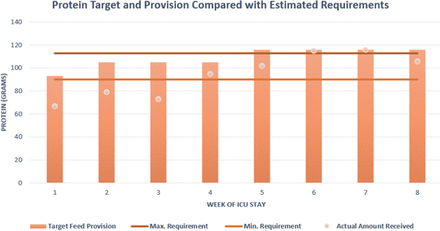
In total, 52 days of nasogastric tube feeding were completed but with notable interruptions between weeks 2 and 4 secondary to proning and imaging necessitating reduction or breaks in feeding. Feed composition was reviewed throughout the clinical course and switched dependant on fluid restrictions and gastrointestinal tolerance of which the former was more problematic, notably in the initial 2 weeks of admission when contributions from intravenous fluids were more significant. The preferred feed choice of high energy/protein feeds (1.3 kcal/mL) inclusive of fibre was most consistently used to meet estimated nutritional requirements. Periodic changes to a more concentrated feed (2 kcal/mL) were adopted to optimise provision and avoid caloric deficit during periods when proned ventilation was not required. Figures 2 and 3 illustrate weekly averages of energy and protein, respectively, indicating target provision versus actual amounts received in the context of the estimated requirements. Note that the initial 3 weeks include propofol provision deemed nutritionally significant averaging >200 mg/hour to 264 kcal/day. Thereafter the primary source of sedation was midazolam with limited propofol and has therefore not been included as part of total energy provision.
Energy target (grey bar) and actual provision (orange dot) compared with calculated estimated requirements of energy given as a minimum and maximum value. ICU, intensive care unit.
Protein target (pink bar) and actual provision (pink dot) compared with calculated estimated requirements of protein given as a minimum and maximum value. ICU, intensive care unit.
It proved difficult to ensure both caloric/protein provision met requirements throughout this critical care admission. Initially between weeks 1 and 3, energy provision includes the caloric contribution from propofol (calculated at 1.1 kcal/mL) and ranged from 180 to 280 mg/hour—averaging just above 200 mg/hour for this time period providing on average an additional 264 kcal/day. Combining this to actual feed provided energy target was kept above the minimum calculated requirement, however, issues with meeting the target rate of feed meant that total feed delivered fell under the prescribed total. This is reflected in the provision of protein which conversely did not meet the target requirements until week 4. Many factors contributed to the observed deficit, for example, fasting secondary to investigations, proned ventilation and the patient removing tubes when sedation was reduced and displaying intermittent periods of agitation. Delays in feed provision were also observed due to time spent awaiting NGT placement confirmation as, often, radiographic evidence was required. Five of the 13 chest radiographs taken during this admission were specifically requested to assess tube placement with a maximum delay noted as 14 hours from imaging to report as the unit required verification from a consultant or the formal radiology report to be available. A subsequent effect of delays from confirming the position of the nasogastric tube to reinitiating feeding meant that the target feeding rate was not always administered, further contributing to reduction in total nutritional provision.
During the earlier course of the admission, mild/moderate gastrointestinal symptoms were observed secondary to side effects of sedative and analgesic medications required for prolonged ventilation and also antibiotic therapy. The most common observation was cyclical phases of bowels being loose and then experiencing 3–8 days of constipation with evidence of abdominal distension requiring management in the form of laxatives/enemas. Gastric aspirates remained minimal despite proning and significant medication burden and thus routine prokinetics were not required for prolonged periods of time.
Outcome and follow-up
Severe weight loss was evidenced during this prolonged ICU admission with a conservative estimate of 19 kg across the 52 days equating to 24% of initial body weight (although by patient reported weight this estimate could increase to 28%). Evidence of deconditioning was noted on physical examination post ICU stepdown notably a visual reduction in subcutaneous fat at the triceps, chest and muscle wasting at the biceps, clavicle and lower limbs. The patient reported reduced appetite and poor oral intake and had been commenced on a modified texture diet post decannulation. He continued enteral tube feeding for a further 2 days post ICU and trialled various oral nutritional supplements. Despite oral intake contributing to <30% of predicted nutritional requirements, the patient remained adamant to have his tube removed citing discomfort, anxiety and wanting to gain some autonomy of his medical care. He remained in hospital for a further 10 days post ICU stepdown receiving input from physiotherapy, speech and language therapy and ongoing dietetic support. This included information on fortified diets and oral nutritional supplements to encourage weight gain being mindful of incremented gains alongside engagement with physiotherapy. As per the local stepdown pathway, the patient was invited to attend a virtual clinic run by a critical care consultant to assess or signpost if any further support is required.
Discussion
In this case, there are two apparent themes contributing to the extensive weight loss observed leading to malnutrition. First, the direct impacts of critical illness and associated cachexia and, second, the environmental effects associated with changes to the ICU during the pandemic response, which created unforeseen challenges to dietetic assessment, management and importantly nursing care provision.
At the time of writing, this patient represents one of the initial cohort that was admitted to this tertiary critical care unit. This point alone highlights the challenges faced when managing a patient through ‘uncharted territory’ whereby no specific COVID-19 guidelines had been ‘tried and tested’ thus relying on newly synthesised recommendations based on expert consensus.5 6 In line with this, early enteral tube feeding was established and initial nutritional goals targeted hypocaloric feeding secondary to haemodynamic instability, extensive sedation and reliance of mechanical ventilation. This equated to feeding rates targeting 15–20 kcal/kg of actual body weight as per predictive equations recommended which were titrated up throughout the course of admission to 20–25 kcal/kg. The above is in keeping with routine nutritional management accounting for the ebb and flow phase of critical illness appreciating the deleterious impacts of over and underfeeding patient.2 8 10
Since the start of the pandemic further data and research indicates how the above practice could be altered despite at the time being ‘current guidance’ at the time. The LEEP-COVID study provides insights into the metabolic changes and energy expenditure observed in patients with COVID-19.4 This study group is currently assessing the metabolic effects via indirect calorimetry and observed that, after day 7, patients transition to a significantly prolonged hypermetabolic state. Furthermore, at week 3 resting energy expenditure (REE) increased to 29 kcal/kg with a range of increments of 120%–200% to total REE. The implications of this would require significant adjustments of predicted equations used in this case. Considering these findings, caloric provision for our patient would have been far below expected energy expenditure if a similar hypermetabolic state was to be observed and could have been as high as a deficit of ~600 kcal/day. In the absence of such knowledge, the rationale at the time of review was opting for commonly accepted hypocaloric feeding in the initial week and increasing feed provision in an incremental fashion beyond this. In both cases, a sustained proinflammatory state, characteristic ‘cytokine storm’ and consequent catabolic effects highlight the importance of ensuring adequate nutritional provision to lessen malnutrition and sarcopenic changes which have been observed.
In the mid-latter part of the clinical course (weeks 5 and 6), despite upward titration of estimated requirements, we note that caloric provision was below the lower estimated value. Issues contributing to underfeeding at this time point included interruptions in feed due to episodes of proning, investigations necessitating fasting and tube placement issues, as described earlier. ESPEN and the BDA advocate for supplementary feeding via the parenteral route if suboptimal calorie and protein provision is sustained for greater than 1 week or where enteral feeding is not tolerated. In retrospect, this issue was persistent for two consecutive weeks in this case. Another notable challenge was providing adequate protein, which is particularly important as low protein provision in the ventilated patient is associated with higher mortality risk.11 Protein targets were achieved by week 4 thus indicating a deficit in the ebb but also for part of the flow phase of disease. In order to address this challenge across the unit further high protein specific supplements had been authorised for use.
This also demonstrates that the logistical challenges presented during the initial influx of the COVID pandemic, which cannot be underestimated. Ensuring the safe administration of supplementary feed necessitates appropriate nursing staff numbers to facilitate the prescribed nutritional care. During the first wave of the pandemic, nurse:patient ratios were severely impacted and went beyond the gold standard of 1:1 care, often increasing to 1:4 to cope with the growing number of patients and thus prioritising other life sustaining treatments. In view of this initial practice, parenteral nutrition was reserved where severe gastrointestinal issues presented with concurrent evidence of malabsorption or poor feed tolerance, that is, high gastric residual volumes or evidence of gut ischaemia. This provides an insight of feeding practices at the time of this case study, whereby with time units have evolved their COVID critical care practice, enabling more robust adherence to recommended guidelines.
A further consideration would be the role of micronutrients and ensuring adequate provision during this prolonged stay on critical care. In view of the anticipated feed disruptions and inaccuracies of weight loss histories, a local policy was implemented which included the provision of a vitamin preparation supplementing vitamins B and C (Pabrinex) given to all new patients on admission for their initial 10 days. This was to help prevent electrolyte disturbances relating to refeeding syndrome and enabling optimal provision of water-soluble vitamins, also factoring in temporary deficits from enteral feed provision. In the latter part of the patient’s admission (week 6), vitamin D levels were measured and found to be insufficient (34 nmol/L with correlating CRP of 4 mg/L). At this time point, his inflammatory markers had normalised and thus likely provides a truer reflection of status, acknowledging that critical illness and the proinflammatory response make interpretation of micronutrients less reliable.12 Notable in this case, levels of insufficiency are not surprising as vitamin D deficiency remains widespread across the UK especially in the winter months whereby endogenous production is at its lowest and with specific population groups more adversely effected.13 Government guidance identifies the BAME community and individuals with darker skin colour ‘high risk of vitamin D deficiency’ whereby data from the UK Biobank indicate individuals of Black African ancestry are at significant risk of vitamin D deficiency in this season (38.5%).14 More recent literature also suggests a potential role of vitamin D status and COVID-19 specific to the critical care cohort. A retrospective meta-analysis from American and European cohorts associated low vitamin D status with higher rates of ICU admission in COVID-19 with poorer prognosis.15 This further highlights this well recognised association of low vitamin D status within this high-risk ethnic group and the potential impact on COVID-19 severity. Across the pandemic, UK guidance continues to advocate for avoidance of deficient levels and to supplement in these high risk groups.16 In this case, this was not acutely corrected but post discharge advice for replacement was provided to be overseen in primary care.
Other environmental factors and logistical challenges during the pandemic severely impacted dietetic assessment and subsequent management. Hospital recommendations for remote assessments were employed to minimise footfall to the unit and reduce the need for additional PPE. This inherently hindered physical assessments and meant a reliance on the medical and nursing staff to conduct, comment and document on anthropometric measures to help guide feed provision and dietetic management. Barriers to this included equipment availability, time pressures, strained working when wearing PPE and prioritisation of tasks. In our case, weight was based on initial estimation at admission with no subsequent measures until discharge from the unit. No alternative anthropometric data were collected impacting monitoring of nutritional interventions. However, this was not unique for this patient and reflected the level of work intensity and burden across the unit during this unprecedented time. Attempts were made to obtain information from the patient’s primary care records but this patient had no comorbidities and thus limited interaction with healthcare services prior to admission.
Various pathways were implemented to help minimise nutritional losses and prevent deficits such as proning guidelines to manipulate feed timings, staff training around gastric residual volumes and using higher protein content supplements.5 9 Furthermore, a more targeted approach to help to optimise resource management may be of use in the future. For example, research on combining the Prognostic Nutritional Index has shown to predict outcome in this patient cohort.17 This uses serum albumin and lymphocyte levels giving an objective measure of nutritional status and inflammation helps to risk stratify individuals. By identifying higher risk patient’s specific to critical care and COVID-19 this would help focus resources required for individual assessment and tailored management helping departments with workforce management.
This case highlights the needs to act on the many lessons which have been learnt since the start of COVID-19 pandemic. Evolving insights into the pathophysiology and metabolic consequences of COVID-19 continue to better equip clinicians such as common use of medications with proven efficacy such as corticosteroids and antivirals. However, the metabolic and nutritional effects can persist such as hypermetabolism, weight loss, taste changes and reduced appetite, thus ongoing work is required to optimise nutritional status to across the clinical course to help mitigate these effects.
Patient’s perspective
I was surprised to see how much my body has changed and how much weight I’ve lost. My legs and arms are especially bad and I’m really weak; as you can see I can still barely write this form (consent form). When coming to the ward the dietitian and doctors encouraged me to continue to have the feeding tube in for longer but I was desperate to have it out. I feel it was stopping me from fully eating and I was taking the supplement drinks to help. It’s hard as I’m not a big food person but my appetite is slowly getting better every day and I can finally sleep which has helped.
Learning points
To be hypervigilant of the direct and indirect impacts of COVID-19 and nutritional status and not to underestimate the potential catabolic effects leading to malnutrition.
Consider a lower threshold to commence supplementary parenteral nutrition to help achieve nutritional requirements which in turn may help mitigate weight loss in line with evidence suggesting a prolonged hypermetabolic phase.
Assess for micronutrient deficiencies early and especially those identified at higher risk (eg, Black, Asian and Minority Ethnic communities), aiming to correct identified deficiencies and ensure wider sufficiency as per updated clinical guidance.
Address logistical challenges considering ‘enhanced dietetic roles’ which could support nursing staff doubling up to obtain anthropometric measures and physical assessments.
Address local protocols which are susceptible to impact feed delivery, for example, managing gastric residual volumes and methods confirming nasogastric tube (NGT) placement to help prevent time loss administering feed.
Ethics statements
Acknowledgments
I would like to acknowledge the collaborative efforts and work by members of the NNEdPro COVID-19 Taskforce; Dr Dominic Crocombe, Professor Sumantra Ray and Professor Martin Kohlmeier for the critical appraisal process and scientific advising of this case report. I would like to also acknowledge Aisling Phelan RD, Jacqui O’Flynn RD and Intensive Care Consultant Dr Robert Broomhead for their appraisal, clinical care and data collection with regards to this case.
Footnotes
Twitter @DrTimothyEdenRD, @ShaneMacZ
Contributors TE took responsibility for the data and information used in the write-up of this case study. SM contributed significantly to the write-up of this case study using his expertise as a registered dietitian.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.