Article Text
Abstract
Objective While maternal fish consumption in pregnancy has consistently been linked to better cognitive and emotional outcomes in children, fish is also a primary source of exposure to methyl mercury (MeHg), which has been linked to poorer child cognitive outcomes. The aim of this study was to evaluate the associations between MeHg exposure, using calculated MeHg exposure from maternal diet and total mercury (Hg) concentration in maternal blood during pregnancy, and child internalising and externalising behaviours at 3 and 5 years of age.
Design and participants The study sample comprised 51 238 mother–child pairs in the Norwegian Mother, Father and Child Cohort Study. Data on maternal blood Hg concentration in gestational week 18 were available for a sub-sample of 2936 women. Maternal MeHg exposure from diet was calculated from a validated Food Frequency Questionnaire answered in mid-pregnancy. Mothers reported children’s emotional behaviour at age 3 and 5 years by questionnaires including twenty items from the Child Behaviour Checklist. Longitudinal associations were examined using generalised estimating equations, adjusted for potential confounders and stratified by maternal fish intake.
Results Maternal blood Hg concentration (median=1.02 µg/L, 90th percentile=2.22, range=0–13.8) was not associated with emotional behaviour in children. Increasing dietary MeHg intake (median 0.15 µg/kg body weight/week, 90th percentiles=0.31, range=0–1.86) was significantly associated with lower internalising β=−0.03 (95% CI −0.05 to –0.00) and externalising child behaviours β=−0.04 (95% CI −0.07 to –0.02) in adjusted models. The inverse associations were also apparent when stratifying by low/high maternal fish intake (<400 and ≥400 g/week).
Conclusions The results indicated that prenatal MeHg exposure, well below the weekly tolerable intake established by European Food Safety Authority (1.3 µg/kg bw), did not adversely affect child emotional regulation. Children of mothers consuming fish regularly were less likely to show signs of emotional behavioural problems.
- mental health
- nutrition assessment
Data availability statement
Data are available on reasonable request. The consent given by the participants does not open for storage of data on an individual level in repositories or journals. Researchers who want access to data sets for replication should apply through helsedata. Access to data sets requires approval from the Regional Committee for Medical and Health Research Ethics in Norway and an agreement with MoBa.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Maternal diet plays an important role in child development.
Fish consumption is recommended as part of a healthy diet and contributes key nutrients but is also a source of exposure to environmental contaminants, including mercury.
WHAT THIS STUDY ADDS
We explored prospective associations between prenatal exposure to mercury and maternal fish intake with child emotional regulation up to 5 years.
The results showed that maternal fish intake and associated prenatal mercury exposure below tolerable intakes were associated with fewer behavioural problems in children.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The study supports findings from other studies showing no adverse effects of prenatal mercury exposure on child emotional regulation when the source of mercury is fish containing relatively low concentrations of mercury.
Introduction
Diet is the main contributor to mercury (Hg) exposure in humans, and the main food source is fish and other seafood.1 Population studies consistently show that fish intake correlates with Hg concentration measured in biological samples,2 3 including during pregnancy and in cord blood samples.4 Large predatory fish species at the top of the food chain contain the largest quantities.
Birth cohort studies have investigated the potential effects of prenatal Hg exposure, resulting from a maternal diet including fish and seafood with varying Hg levels, on subsequent child development. The findings from two pioneer Hg cohort studies conducted in populations with high, but varied, seafood diets reported conflicting results concerning the magnitude of prenatal Hg exposure that may cause impaired childhood neurodevelopment, performance and behaviour.5–9
Studies in populations with a moderate intake of fish, defined as 1–3 servings of fish per week and a lower exposure level of Hg also show variation in the association to child emotional development.10–14
Previous research in large cohort studies has indicated that healthier maternal dietary patterns and diet quality assessed during pregnancy are related to better emotional and cognitive health in children over the first years of life.15 These relationships persist even when considering the potential confounding effect of familial and maternal factors, such as mothers’ mental health, parent education level, income, marital status and parenting styles.16 The meta-analysis by Borge et al,16 which included data from a large pregnancy cohort in Norway, found relatively small effect sizes and indicated a slightly larger effect size for the cognitive than for the affective domain.
Previous studies in the Norwegian cohort have shown mixed findings for Hg exposure and health outcomes. Hg exposure in pregnant women was associated with delayed language and communication skills in children at age 3 years, OR 2.22 (95% CI 1.31 to 3.72),17 but with favourable child language outcomes at 5 years.2
The aim of this study is to follow up the previous findings in the cohort and assess if there is a relationship between higher prenatal Hg exposure and adverse child emotional health outcomes. We will investigate the associations between Hg concentration in maternal blood during pregnancy, and calculated MeHg exposure from maternal pregnancy diet, and associations with child internalising and externalising behaviours at 3 and 5 years of age from the Child Behaviour Checklist (CBCL).
Material and methods
Data sources
The Norwegian Mother, Father and Child Cohort Study (MoBa) is a prospective population-based pregnancy cohort conducted by the Norwegian Institute of Public Health (NIPH).18 The recruitment period was from 1999 to 2008 and pregnant women and their partners from all parts of Norway were invited to the study. Mothers could participate with more than one pregnancy and the cohort includes 95 200 mothers and 114 500 children. Of the invited pregnant women, 41% consented to participation. All participants gave written informed consent at recruitment.
Study population
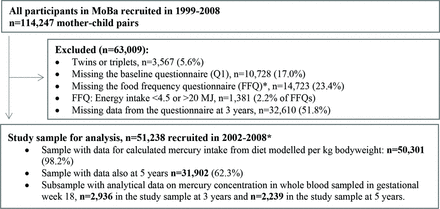
This study is based on MoBa datafile V.9 released for research in 2015. The dataset is linked to data from the Medical Birth Registry of Norway a national health registry containing information about all births in Norway. The questionnaires used in the current study were sent out in gestational week 15 (Q1) and 22 (Q2), at 6 months after birth (Q4), and when the child reached age 3 years (Q3y) and 5 years (Q5y). Q2 is a Food Frequency Questionnaire (FFQ), while Q1, Q4, Q3y and Q5y are general questionnaires covering maternal and child health, lifestyle and background factors. We excluded participants with multiple births and mothers reporting unlikely energy intake (<4.5 or >20 MJ). The final study population included 51 238 women with singleton live births who had answered questionnaires Q1, Q2, Q3y, of whom 31 902 also had answered the questionnaire Q5y (figure 1).
Flow chart of inclusion. *The MoBa food frequency questionnaire was introduced in March 2002. MoBA, Norwegian Mother, Father and Child Cohort Study.
Exposure
Pregnant women in MoBa completed a comprehensive semi-quantitative FFQ designed to capture their average dietary intakes since the start of pregnancy. The FFQ included questions about intake of 255 food items with special emphasis on various seafood items. Food frequencies were converted to food amounts, and food and nutrient intakes were calculated with use of the FoodCalc program19 and the Norwegian food composition table.20 A validation study in 119 MoBa participants showed that the FFQ is a reliable and useful tool for estimating the intakes of nutrients and foods, including fish and seafood.21 22
Concentrations of total Hg in Norwegian fish and other food items were compiled in a database described previously.23 Prenatal dietary Hg exposure was calculated by combining reported food intakes with this database. MeHg is the organic and most toxic form of mercury, constitutes 80%–100% of the total Hg in seafood. Seafood contributes 88% of the total Hg dietary exposure among women in MoBa, and we considered Hg from seafood to reflect MeHg exposure. Total fish intake was calculated as the sum of lean and oily fish species, reported as intakes in the form of bread spread, salad, mixed dishes and dinners (including only the fish part of processed fish items and mixed fish dishes). To perform stratified analysis, we used a cut-off at 400 g of fish intake per week. The rationale for this cut-off is the recommendation by the Norwegian Directorate of Health for a weekly fish intake of 300–450 g.24
Maternal whole blood specimens were obtained around 18 weeks of gestation. The samples were stored at the MoBa biobank. Maternal whole blood samples from 3000 individuals were retrieved from the biobank in 2015 for the Human Environmental Biobank project (MoBa Etox) at NIPH.25 Maternal blood concentrations of Hg were made available for this study. In total, n=2936 individuals in the MoBa Etox sample had data from the questionnaire at 3 years and n=2239 also had data from the questionnaire at 5 years. Mercury analysis was conducted at the Department of Laboratory Medicine at Lund University (Sweden) and was determined as total mercury in acid-digested samples by cold vapour atomic fluorescence spectrophotometry. For a more detailed description of the measurement method, see our previous paper.2
Outcome
The primary outcome of this study was maternal report of child emotional behavioural regulation assessed by the CBCL included in the 3-year and 5-year questionnaire. CBCL is a widely used method of identifying internalising symptoms of anxiety and depression and externalising symptoms of aggressive behaviour and attention problems in children and is the most renowned parental report tool of behavioural problems for young children. MoBa questionnaires at 3 and 5 years include a selection of 26 items from the original CBCL. Adequate sensitivity (71%) and specificity (92%), for prediction of psychiatric disorders using CBCL was found in Norwegian study.26 Latent factor analysis of the MoBa subset of CBCL questions on the two expected constructs of internalising and externalising was found to correlate 0.92 with the full original scale.27 CBCL has been used to assess the effect of MeHg on emotional behaviour in previous studies.28
The CBCL in MoBa consisted of 9 items of internalising behaviours (min 9, max 21) and 10 items of externalising behaviours (min 10, max 30) (for information on items see online supplemental table S1). The mother assesses statements about the child behaviour with three response options: ‘true’, ‘somewhat or sometimes true’ and ‘not true’. For the purpose of this study, the scale is presented such that higher scores represent more internalising and externalising behaviours.
Supplemental material
Covariates
Covariates considered as potential confounders were selected a priori on theoretical grounds. The following variables were obtained from the baseline questionnaire answered around pregnancy week 17: prepregnancy bodymass index was calculated from self-reported weight and height and categorised according to WHO categories, level of educational, smoking, annual income and maternal symptoms of anxiety and depression in early pregnancy assessed by the Hopkin Symptom Checklist (HCBL-5). The following variables were obtained from the medical birth registry: age at delivery and parity dichotomised as primiparous and parous.
Statistics
The same questionnaire items and exposure measurements were used at 3 and 5 years. Generalised estimation equation (GEE) regression models were used for estimating association between mercury exposure and internalising or externalising behaviour scores after accounting for potential confounders. The GEE models assumed a Gaussian distribution for the dependant variables and employed an unstructured covariance pattern to account for within participants autocorrelation in internalising/externalising behaviour scores across age 3 and 5 years assessments. Missing data in the CBCL scores were imputed by using scores within the same dimension. There had to be at least one value per dimension, or it was coded as system missing. For the covariates, we used a complete case approach and excluded participants with missing data in the covariates.
Prenatal dietary Hg exposure calculated from maternal diet and maternal Hg blood level measurement was used as a continuous exposure. In addition, reported fish intake was dichotomised using ≥400 g per week defined as a high intake to construct a nominal high and low intake exposure variable. Secondary analyses were performed using this dichotomised exposure. A nominal measurement time was included in the regression equation and the main effect of mercury exposure average across age 3 and 5 years measurements was investigated. Three models were employed to account for potential confounders. Model 1 adjusted for maternal age, maternal education and income; model 2 additionally adjusted for prepregnancy body mass index (BMI), maternal smoking, parity and model 3 made further adjustment for maternal symptoms of anxiety and depression during pregnancy.
All analyses were performed using IBM SPSS Statistics V.23 for Windows (SPSS). All p values were two sided and values below 0.05 were considered statistically significant.
Results
Total blood mercury (µg/L) was available for n=2936 pregnant women. The median maternal blood Hg concentration was 1.02 µg/L (10th and 90th percentiles; 0.34, 2.22 µg/L) (range 0–13.8 µg/L).
Of the 51 238 mothers who had answered the questionnaire at child age 3 years, 31 737 (62%) had also answered the questionnaire at 5 years. Comparison of key maternal characteristics and prenatal Hg exposure by the two time points indicated no notable difference for key characteristics or for dietary MeHg exposure and blood Hg concentration for drop-outs and non-drop-outs (online supplemental tables S2–S4).
In the study sample (n=51 238), the mean age of the women at the time of delivery was 30 years and the mean prepregnant BMI was 23.8 kg/m2. Most women (72%) reported to have attained at least one or more year of college/university, 93% of the women did not smoke during pregnancy and nearly half (48.7%) were nulliparous.
The median dietary MeHg exposure was 0.15 µg/kg bw/week (10th and 90th percentiles; 0.06, 0.31 µg/kg bw/week (range 0–1.86 µg/kg bw/week). The blood Hg concentration and calculated dietary MeHg exposure correlated moderately; Spearman rs=0.37 (95% CI 0.34 to 0.40). Both dietary MeHg exposure and blood Hg concentration increased with increasing fish intake (online supplemental table S3).
The median maternal weekly fish intake was 181 g/week with 10th and 90th percentiles at 57 and 366 g/week (range 0–1850 g/week). The participants were divided into low or high fish consumers, based on intakes <400 g per week or ≥400 g per week. Only 7.3% (n=3722) of the women reported eating 400 g of fish or more per week.
Maternal fish intake (n=51 238) correlated strongly with MeHg calculated from the diet (Spearman rs=0.88, 95% CI 0.88 to 0.88) and moderately with maternal blood Hg concentration (n=2936, r=0.36, 95% CI 0.33 to 0.39).
The CBCL scores for internalising and externalising child behaviour at 3 and 5 years are described separately and by maternal fish intake below and above 400 g per week (table 1). The group of mothers who reported a weekly fish intake ≥400 g reported slightly lower scores for child emotional behavioural problems at both 3 and 5 years.
Distribution of mean CBCL scores at children aged 3 and 5 years and by maternal reported weekly fish intake below and above 400 g
In the analysis with prenatal dietary MeHg as the exposure, MeHg intake was associated with lower internalising and externalising problem scores in both crude and adjusted models. We adjusted for covariates in the models by adding more variables into the analysis in three steps. The first model included maternal age, education and income. In model 2, we included prepregnancy BMI, smoking and parity, and in the third full model we included maternal anxiety and depression (HCBL-5) during pregnancy. The effect estimates were attenuated by the adjustment but remained significant in the final model with beta=−0.03 (95% CI −0.05 to –0.00) for internalising behaviour and beta=−0.04 (95% CI −0.07 to –0.02) for externalising behaviour (table 2). When using maternal blood Hg concentration as the exposure variable, the effect estimates indicated no associations with child emotional behavioural outcomes in either crude or adjusted analyses (table 2).
General estimating equation models and association between maternal dietary Hg intake during pregnancy and maternal Hg blood concentration as continuous variables and maternal report on child internalising and externalising behaviour problems adjusting for maternal covariates in additive models
We performed stratified analyses by maternal weekly fish intake below and above 400 g and found the same direction of associations within both groups. Within the low fish intake group, we found that higher dietary MeHg exposure was associated with lower scores for internalising and externalising problems in both unadjusted and fully adjusted models. In the much smaller high fish intake group, the associations indicated similar a direction and effect size but the confidence intervals were wide (table 3).
General estimating equation models and association between maternal dietary Hg intake during pregnancy and maternal Hg blood concentration as continuous variables and maternal report on child internalising and externalising behaviour problems
Discussion
In this study, we found that MeHg exposure calculated from maternal diet (median 0.15 µg/kg bw/week) was associated with fewer emotional behavioural problems in children at age 3 and 5 years. This inverse association was evident across both lower and higher intakes of seafood consumption. Prenatal maternal blood Hg concentration (median 1.02 µg/L) was not associated with increased child emotional behaviour problems in the subsample with available blood Hg values.
The dietary MeHg exposure level in MoBa was generally below the current tolerable weekly intake of 1.3 µg/kg bw established by the European Food Safety Authority (EFSA), which is derived from hair mercury concentrations at the no-observed-adverse-effect level in populations in the Faroe Islands and Seychelles (11.5 mg/kg maternal hair).29 Using a hair:blood ratio of 250 and an uncertainty factor of 2 to account for variability in this ratio, the maternal blood concentration corresponding to this hair level is 23 µg/L. The majority of women in MoBa had low exposure levels, with only a few individuals with calculated dietary MeHg values exceeding the tolerable weekly intake and none with exceeding Hg concentrations in blood. The median maternal blood Hg concentration was 1.02 µg/L (10th and 90th percentiles; 0.34, 2.22 µg/L) (range 0–13.8 µg/L).
There is a considerable variation in Hg exposure levels worldwide due to variability in the diet and/or due to specific local contamination. In populations with a traditionally high fish intake, we find higher levels of Hg in blood than in populations with a moderate fish intake. The geometric mean blood Hg concentration in the Republic of Korea was 3.2 µg/L,30 while in US women the mean blood Hg concentration was approximately 1.0 µg/L.1
In earlier studies in MoBa, we have shown that both the calculated dietary MeHg exposure and maternal blood Hg concentration increases with increasing fish intake.2 Investigating possible effects of prenatal Hg exposure on child development, we found that prenatal dietary exposure above the 90th percentile (>0.29 µg/kg bw/day) was associated with impaired language development in children aged 3 years, indicating that for the subgroup with the highest Hg exposure, adverse effects of Hg exposure could outweigh the beneficial nutritional effect of maternal seafood consumption.17 However, in a subsequent study that focused on the MoBa children at 5 years of age, the observed associations between prenatal dietary MeHg exposure and child language and communication skills were favourable, suggesting that the benefits of fish consumption outweighed the potential negative effect of the low MeHg exposure.2 Another explanation could be that the parental reported assessment of language and communication skills in children is more accurate and reliable at 5 years than at 3 years.
Our findings are in line with those from the large population cohort in the UK, ALSPAC, which found that maternal blood Hg (median 1.86 µg/L) was positively associated with child development, behaviour and scholastic performance when the mother ate fish, whereas the associations were null or negative when the mothers did not eat fish.31 Similar results were also found in the INMA study in Spain among populations with high fish consumption. Here they found that both increasing cord blood Hg concentration (geometric mean 8.8 µg/L),32 and maternal fish consumption above the recommended limit of 340 g/week, were associated with higher scores on neuropsychological tests.33 A study from the New Bedford cohort in the US found that maternal hair Hg (median 0.45 µg/g) was associated with elevated risk of attention-deficit/hyperactivity disorder (ADHD) -related behaviour. However, maternal fish consumption during pregnancy (> 2 servings per week) was inversely associated with ADHD-related behaviour.14 These studies support the hypothesis that nutritional benefits are larger than the potential harm from exposure to Hg from seafood intake within the recommended levels.
The main strengths of this study include the large cohort of mothers and children from all parts of Norway, the detailed data on maternal diet and lifestyle, and the prospective design, which limits the problem associated with recall bias and reverse causality. We assessed Hg exposure using both Hg concentration in blood and calculated dietary MeHg, and both markers of Hg exposure increased with increasing seafood intake. Child behaviour was assessed using a well-established and widely used tool and longitudinal modelling minimises the impact of measurement error. This is a more robust method than studying associations between Hg exposure and outcomes for each time point separately.34 An important limitation of this study is that dietary intake, covariates and child outcomes were assessed by maternal report. Self-reported data on health-related and lifestyle-related information pose many challenges.35 Use of FFQ to assess dietary intake is a rather crude method and prone to misreporting. Although the FFQ has been extensively validated, misreporting and imprecision is likely to have attenuated the effect estimates.36 The low response rate in MoBa is also of concern. Participants in MoBa were slightly older, leaner, better educated and included fewer smokers than the general population of pregnant women in Norway at the time, but examination of several exposure–outcome associations in MoBa found comparable effect estimates in MoBa and a nationally representative study sample.37 It is possible that the beneficial association between fish intake and emotional behavioural scores could be confounded by other dietary components. However, we did not adjust for other aspects of overall diet because nearly all women in MoBa reported fish consumption.38 Moreover, fish intake is not strongly associated with diet quality in women participating in MoBa.39
Although we adjusted for numerous variables determined a priori, the study is observational and the possibility of residual or unmeasured confounding cannot be excluded. The loss to follow-up is another concern and in this study nearly 40% of mother–child pairs were lost to follow-up age 3–5 years. Although participants who remain cohort members differ from those who drop out, studies in MoBa have shown that this does not severely bias the results.40 41
The potential biological mechanisms for the beneficial effects of seafood have been attributed primarily to the marine long chain fatty acids.8 However, fish also contributes to the intake of high-quality protein and has essential elements such as iodine and selenium. It is not clear, however, whether the developmental benefits ascribed to eating fish are explained only by nutrients or could also be due to healthier lifestyles that often correlate with regular fish consumption. Overall, results from experimental and prospective observational studies conclude that moderate intake of fish species low in mercury is associated with lower risk of morbidity and mortality, including mental health, and more favourable pregnancy outcomes including child neurocognitive development.42 The results of the current study support the need to promote fish consumption in women of childbearing age in accordance with country-specific warnings to restrict consumption of fish species known to contain high MeHg and other contaminants.42
Exposure to Hg in women of childbearing age continues to be an important public health concern, and even though the exposure to Hg from fish and seafood in pregnant women in Norway is generally low, some fish and fish products from other areas of Europe contain Hg levels exceeding those recommended for consumption by pregnant women.43 The fish species most commonly consumed in Norway are cod and farmed salmon, which contain low levels of Hg. Our results indicate no association between Hg exposure and child behaviour of clinical significance in the MoBa cohort. However, it has been argued that cognitive disorders are increasing worldwide44 and low-level environmental toxicants may contribute to subclinical decrement in brain function that can have severe consequences for individual life quality and on population level.45
Conclusion
In summary, this study supports findings from other studies showing no adverse effects of prenatal Hg exposure on child emotional regulation when the source of Hg is fish containing relatively low concentrations of Hg resulting in an intake well below the tolerable intake set by EFSA. Children of mothers who consumed fish regularly had lower scores for emotional regulation problems than those of mothers reporting low fish intake (< 400 g/week). These findings corroborate existing dietary guidelines encouraging women of childbearing age to include fish regularly as part of a balanced diet, while restricting consumption of fish species with high concentration of mercury or other contaminants.
Data availability statement
Data are available on reasonable request. The consent given by the participants does not open for storage of data on an individual level in repositories or journals. Researchers who want access to data sets for replication should apply through helsedata. Access to data sets requires approval from the Regional Committee for Medical and Health Research Ethics in Norway and an agreement with MoBa.
Ethics statements
Patient consent for publication
Ethics approval
The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from The Regional Committees for Medical and Health Research Ethics. The MoBa cohort is now based on regulations related to the Norwegian Health Registry Act. The current study was approved by The Regional Committees for Medical and Health Research Ethics (REK South-East A 2015/1346).
Acknowledgments
We are grateful to all the participating families in Norway who take part in this on-going cohort study.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors FJ initiated the study and obtained funding for data acquisition. All authors contributed to planning the study. MH, KV and A-LB contributed with acquisition of the data, calculation of mercury exposure and project administration. FJ, MM and KV conducted the statistical analyses. KV wrote the first draft of the manuscript. All authors contributed to interpretation of the results and critically reviewed the manuscript. All authors read and approved the final manuscript. FJ and KV are responsible for the overall content as guarantors.
Funding The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. FJ is supported by an NHMRC Investigator Grant (#1194982). She has received: (1) competitive Grant/Research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, The University of Melbourne; (2) industry support for research from Meat and Livestock Australia, Woolworths Limited, Bega Cheese, the A2 Milk Company, Be Fit Foods; (3) philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, the Waterloo Foundation and (4) travel support and speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Network Nutrition, Angelini Farmaceutica, Eli Lilly, Metagenics, and The Beauty Chef. FJ has written two books for commercial publication.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.