Article Text
Abstract
Objective Sarcopenic obesity is a key feature in osteoarthritis (OA). While ideal OA treatment involves physical activity and diet, how diet influences OA pathophysiology is unclear. We explored the associations between diet, nutrition risk and physical activity with body composition in older adults with OA.
Methods Baseline data from the Canadian Longitudinal Study on Aging data set were analysed. Participants with hip, knee, hand or multiple forms of OA were included in this cross-sectional analysis. Body composition measures (lean, fat and total masses (kg) and body fat percentage) were separate dependent variables. Regression analyses were conducted to explore associations between body composition with dietary intake (high calorie snack, fibre), nutrition risk (SCREEN II) and physical activity (Physical Activity Scale for the Elderly).
Results 1596 participants were 66.5 (9.0) years old with a body mass index of 28.2 (5.3) kg/m2. Higher fibre cereal intake was associated with higher lean mass (unstandardised beta coefficient 0.5 (0.1, 0.9), p=0.02) and lower body fat percentage (−0.3 (−0.6, 0.0), p=0.046). Lower nutrition risk was associated with higher lean mass (0.1 (0.0, 0.1), p=0.03), lower fat mass (−0.05 (−0.1, 0.0), p=0.009) and lower body fat percentage (−0.1 (−0.1, 0.0), p<0.001). Higher physical activity was associated with higher lean mass (0.01 (0.01, 0.02), p<0.001), lower fat mass (−0.01 (0.0, 0.0), p=0.005) and lower body fat percentage (−0.01 (0.0, 0.0), p<0.001).
Conclusion Greater physical activity and lower nutrition risk were associated with better body composition. While fibre intake was also associated body composition, the CIs were wide suggesting weak associations.
- musculo-skeletal health
- nutrition assessment
- physical performance
Data availability statement
Data may be obtained from a third party and are not publicly available. This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the CLSA is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation. This research has been conducted using the CLSA data set, Baseline Comprehensive Dataset V.3.1, under Application Number 161014. The CLSA is led by Drs Parminder Raina, Christina Wolfson and Susan Kirkland.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
The loss of lean tissue and accumulation of fatty tissue are linked with worse structural disease and illness experience outcomes in osteoarthritis (OA).
Nutrition risk describes the risk of poor nutritional status across a range from healthy nutrition to malnutrition. Nutrition risk reflects physiological status, the ingestion, absorption and metabolism of foods, along with social, economic and behavioural factors.
WHAT THIS STUDY ADDS
Determinants of body composition point to important therapeutic targets for this disease.
This study explores associations between diet, nutrition risk and physical activity with body composition in OA.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings show that higher engagement in physical activity, lower nutrition risk and, to a small extent higher fibre cereal intake, were associated with better body composition in a sample of 1596 Canadian older adults with OA.
Lifestyle behaviours around nutrition and physical activity are important to consider in OA disease.
Introduction
Osteoarthritis (OA), a joint disease that causes chronic pain, is expected to affect 10 million in Canada by 2041 due to an increasingly older1 and obese2 population. Healthcare costs will rise to $C8.1 billion by 2031.3 Obesity is a key risk factor for OA incidence and worsening.4 5 In fact, a body mass index (BMI) >35 kg/m2 had a 4.7-fold elevated risk of OA.6 OA is linked to sarcopenic obesity; that is, an elevated fat mass with a concomitant reduced lean mass.7 Yet, effective treatment to improve body composition in OA remains elusive.
Body composition is linked to structural joint damage and physical function in OA. Radiographic knee OA was associated with both regional lower lean mass in 4194 Korean adults8 and greater fat mass and fat-to-lean mass ratio in 212 post-menopausal women.9 More sensitive measures of structural OA disease, such as cartilage volume loss captured with MRI, corroborate this radiographic work.10 In 153 adults, a 1 kg greater total body fat mass increased the odds of both knee cartilage defects; while a 1 kg greater muscle mass was related to a larger cartilage volume.11 12 Greater self-reported physical activity is associated with lower fat mass, higher lean mass and skeletal muscle index among older adults.12 Despite this evidence, a large knowledge gap exists in how to effectively improve body composition in this population.10
Concurrently reducing fat mass and maintaining lean mass is an important goal in OA.11 Calorie-restricting diets and/or exercise interventions in OA effectively reduce fat mass—but also concurrently reduce lean mass.13 14 14 This loss of lean muscle may be profoundly detrimental in OA, particularly in weight-bearing joints.15 Lean mass was positively associated with physical function and quality of life.16 Further, strong muscle contraction is necessary to manage dynamic joint loads that contribute to OA worsening over time.15 More refined dietary interventions are necessary than generic calorie restriction. To achieve this specificity in dietary OA interventions, a deeper understanding of malnutrition in OA, and its impact on body composition, is necessary.17
Malnutrition, defined as a deficiency or excess of one or more essential nutrients that creates functional changes, leads to a greater risk for functional impairment, mortality, disease and dependence.17 Among older adults receiving at-home nursing services, >40% were at risk of malnutrition (34.5%) or malnourished (8.1%).18 This nutrition risk influences the presence of sarcopenic obesity.19 Higher fat-free mass index and body fat percentage were associated with a lower likelihood for nutrition risk.20 From this perspective, nutrition risk, which incorporates aspects of diet as well as nutrition behaviour for community-living seniors, may be an important modifier of body composition.17 Despite the high prevalence of obesity in those with OA, the association between nutrition risk, along with diet and physical activity, has received little attention in this population.
The purpose of this study was to determine whether nutrition risk, dietary intake and physical activity level explained variance in body composition among older adults with OA. It was hypothesised that poorer diet (higher intake of high calorie snacks, lower intake of high fibre cereal), higher nutrition risk and lower reported physical activity would be associated with lower lean mass and higher fat mass, body fat percentage and total mass in older adults with hip, knee and hand OA. These findings could point to interventional targets to combat sarcopenic obesity in OA.
Methods
Sample
This research was conducted using baseline data from the Canadian Longitudinal Study on Aging (CLSA) comprehensive data set.21–23 The CLSA is a national study with 11 participating centres across Canada. Coordinators at each of the data collection sites underwent a 3-day training session at the CLSA National Coordinating Centre, following which they were responsible for training staff at their local data collection site.22 This training ensured that all data collection procedures were standardised across sites. The CLSA aims to study factors related to ageing and adult development with the goal of developing interventions to promote healthy ageing.22 This data set includes a national sample of participants aged 45–85 years with information pertaining to health and lifestyle, while also reporting quantitative outcomes on a variety of physical, psychological and physiological measures. The CLSA comprehensive data set includes data from 30 097 participants recruited through provincial healthcare registration databases, random digit dialling and the Quebec Longitudinal Study on Nutrition and Aging, with collections taking place between May 2012 and 2015.22 Participants in the CLSA must have been willing to participate in both an in-home interview and visit a data collection site.
From the whole CLSA data set, the inclusion criteria for this analysis included participants with diagnosed hip, hand and/or knee OA. Exclusion criteria were neurological or respiratory conditions or injuries (ie, dementia, Alzheimer’s disease, multiple sclerosis, chronic obstructive pulmonary disease), rheumatoid arthritis and clinical depression. These exclusion criteria were applied to reduce the potential confounding effect of chronic disease, for example, by influencing physical activity. Despite the positive association between physical activity and health and the benefits it has on chronic disease, a lower percentage of older adults achieve weekly physical activity recommendations if they have chronic disease (musculoskeletal, stroke, degenerative neurological, vascular/heart, diabetes mellitus, respiratory) compared with those who do not.24 Participants without a complete data set were also excluded.
Food intake and nutrition risk
Food intake variables were measured using the Short Diet Questionnaire. This food frequency questionnaire asks participants to record their usual intake of 36 food and drink items over the past year, from which number of daily servings is calculated. This questionnaire asks participants to record the frequency of food intake by providing a number and then indicating per day, week, month or year. They could also choose to refuse to answer or indicate that they did not know the answer. Two independent variables were obtained from this questionnaire: high calorie snack intake (NUTHC) and high fibre cereal intake (NUTFBR). These two food items were selected because these were hypothesised to be different between obese and non-obese participants. NUTHC was the total consumption of items from four questions of the questionnaire: (1) ice cream, ice milk, frozen yoghurt, milk-based desserts, (2) salty snacks, (3) cakes, pies, doughnuts, pastries, cookies, muffins and (4) chocolate bars.21 The overconsumption of calories, including those from sugars, is associated with increased risk of a variety of health conditions including diabetes, cancer, heart disease and metabolic disease, as well as arthritis.25 A higher NUTHC score reflected poorer quality diet. NUTFBR was a single question from the diet questionnaire regarding intake of high fibre cereal. Higher fibre intake has been related to reduced risk of knee pain.26
Nutrition risk was measured using the 8-item SCREEN II tool.27 This tool designed for community-living seniors determines nutrition risk through questions related to recent weight gain/loss over the past 6 months, appetite, frequency of skipping meals, physical challenges while eating or drinking (ie, coughing, choking), fruit and vegetable, as well as fluid consumption and meal preparation. This tool provides a continuous score (nutrition risk (NUR)) from 0 to 48, with higher scores reflecting lower NUR. Additionally, participants were classified into three categories of nutrition risk (NURCLS): low risk (NUR ≥43), moderate risk (38≤NUR<43) and high risk (NUR <38).27
Physical activity
Physical activity was measured using the Physical Activity Scale for the Elderly (PASE).28 This questionnaire asks participants to report the frequency, type and time of their participation in a variety of physical activities, including recreational, activities of daily living and work in the past week. The total time spent in different activities is then multiplied by individual weighting factors, from which a total PASE score is calculated. PASE scores range from 0 to 793, with a higher score reflecting higher physical activity level.12
Covariates
Covariates included age, sex, BMI, OA type, depressive symptoms and socioeconomic factors.8 16 20 OA type was defined through seven classifications: hand only, knee only, hip only, hand and knee, hand and hip, knee and hip and all three forms. The depressive symptoms variable was defined as the participants’ score on the 10-item Center for Epidemiological Studies Short Depression Scale (CES-D-10) questionnaire, where scores range from 0 to 30, with higher scores indicating greater depressive mood.29 Socioeconomic factors included highest education level, income bracket and self-reported social inequality rating. Highest education level was grouped into the following categories: (1) no post-secondary degree, certificate or diploma, (2) trade certificate or diploma from a vocational school or apprenticeship training, (3) non-university certificate or diploma from a community college, Collège d'enseignement général et professionnel (CEGEP), school of nursing and so on, (4) university certificate below bachelor’s level, (5) bachelor’s degree, (6) university degree or certificate above bachelor’s degree. Income bracket was grouped into the following categories: (1) less than $C20 000, (2) $C20 000 or more, but less than $C50 000, (3) $C50 000 or more, but less than $C100 000, (4) $C100 000 or more, but less than $C150 000, (5) $C150 000 or more. Social inequality rating asked participants to rate their stance within their community by envisioning a 10-step ladder, and asking them to report which step they would place themselves on.
Dependent variables: body composition
Body composition was measured using an Hologic Discovery A dual energy X-ray absorptiometry (DEXA) scanner. DEXA scanning is less expensive with lower radiation exposure than CT, while offering excellent reliability for body compositional measurements.22 30 While several regional body composition measurements were captured in the CLSA, the current analysis studied whole body compositional measurements including total body lean mass (kg), total body fat mass (kg), body fat percentage (%) and total mass (kg).
Data analysis
Regression analyses were performed for each body composition outcome as a dependent variable (lean mass, fat mass, body fat percentage, total mass). For each, six models were created. The initial model included only covariates. For the remaining five models, each independent variable (NUTHC, NUTFBR, NUR, NURCLS, PASE) was added independently to the covariate model. Likelihood-ratio tests were conducted to determine whether each independent variable explained significantly more variance than the covariate model. Further, because NURCLS was ordinal, effect sizes (d) were calculated between NURCLS (low NUR, moderate NUR, high NUR) for each outcome measure. Margins plots were created for statistically significant independent variables. Model diagnostics (collinearity, normality, heteroskedasticity) were assessed and visually inspected. Statistical analyses were performed in Stata/IC V.13.0 (StataCorp, College Station, Texas, USA).
Results
Sample
Of 30 097 participants who completed the baseline in-home interviews and data collection site visits in the CLSA, 5.3% (n=1596 participants) met the criteria to be included in this analysis. These 1596 participants were 66.5 (9.0) years old with a BMI of 28.2 (5.3) kg/m2 and 963 were women and 633 were men. Participant characteristics, including mean values and ranges for covariates, independent and dependent variables are presented in table 1.
Characteristics of 1596 participants whose data were included in these analyses (mean (SD), (minimum, maximum))
Covariate model
Each of the covariate models explained a large amount of variance for each of the body composition outcomes. Specifically, the covariate model explained 83.1% of the variance of lean mass (table 2), 85.3% of the variance of fat mass (table 3), 76.7% of the variance of body fat percentage (table 4) and 87.5% of the variance in total mass (table 5).
Regression models for lean mass
Regression models for fat mass
Regression models for body fat percentage
Regression models for total mass
Food intake: high calorie snacks and high fibre cereal
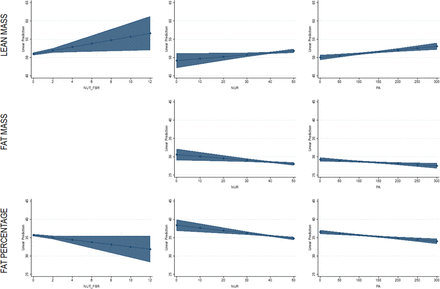
The frequency of NUTFBR was associated with increased lean mass (p=0.02; table 2; figure 1) and lower body fat percentage (p=0.045; table 4; figure 1). NUTFBR was not associated with fat mass (p=0.87) or total mass (p=0.09). The frequency of NUTHC was not associated with any body composition outcomes.
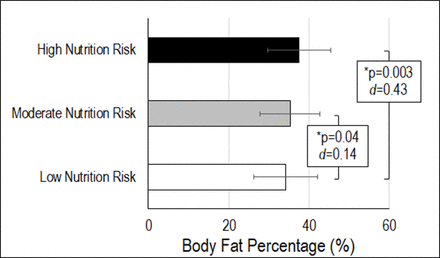
Body fat percentage (%) for each nutrition risk classification (low risk (NUR≥43), moderate risk (38≤NUR<43) and high risk (NUR<38)). Statistical significance (p<0.05) and effect sizes (d) between groups provided. NUR, nutrition risk; NUTFBR, high fibre cereal intake; PA, physical activity.
NUR
NUR was associated with lean mass (p=0.03; table 2; figure 1), fat mass (p=0.009; table 3; figure 1) and body fat percentage (p=0.0002; table 4; figure 1), but not total mass (p=0.94). A higher NUR score (lower NUR) was associated with higher lean mass, lower fat mass and lower body fat percentage. As well, NURCLS was associated with fat mass (p=0.01; table 3) and body fat percentage (p=0.002; table 4), but not lean mass (p=0.31) or total mass (p=0.65). Participants classified as low NUR had less fat mass (p=0.028; d=0.20) and body fat percentage (p=0.039; d=0.14; figure 2) than those at moderate NUR; and less fat mass (p=0.041; d=0.55) and body fat percentage (p=0.003; d=0.43; figure 2) than those at high NUR. Moderate and high NURs were not significantly different for either outcome.
Linear regression margin plots for significant predictor variables (high fibre cereal, nutrition risk score, physical activity): lean mass (top row), fat mass (middle row), fat percentage (bottom row).
Discussion
Lower self-reported physical activity and greater NUR, reflecting nutrition behaviours that are associated with malnutrition, were associated with less lean mass and greater fat mass and body fat percentage in Canadian older adults with OA. This work highlights that NUR is linked to body composition in OA. Further, behaviours around NUR and physical activity level appear more important than the intake of food items, which may be a proxy for diet quality, with respect to body composition in OA. While NUTFBR was associated with higher lean mass and lower body fat percentage, the associations were weak with large CI bands, suggesting other factors influence the relationship between fibre intake and body composition. NUTHC was not associated with any body composition outcomes. Finally, this work emphasises that crude measures of total body mass reflecting obesity do not reveal insight into body composition in OA.
Importance of NUR for body composition in OA
NUR was associated with body composition in this large sample of Canadian older adults with OA. There is no work regarding NUR in OA, and only limited evidence regarding the association between NUR and body composition in older adults. Recently, Chatindiara and colleagues20 showed that lower NUR, measured with the Mini Nutritional Assessment-Short Form (MNA-SF), was associated with higher fat-free mass index and body fat percentage in 257 community-dwelling older adults with a median age of 79 years. These associations may be driven by the inclusion of BMI on the MNA-SF. As a reduction in mass may reflect malnutrition and frailty risk in older adults, researchers suggested a possible protective effect of higher BMI for mortality and morbidity.20 On the other hand, the current analysis showed that in older adults with OA, lower NUR was concurrently related to higher lean mass and lower fat mass and body fat percentage. This conflicting evidence between the current analysis and previous work may reflect differences in NUR tools and in study populations. Also, in the current analysis it is important to note that, among participants with lower NUR scores, the CI bands are wide suggesting lower confidence in estimating body composition in those with greater NUR. Nevertheless, the findings from the current analysis regarding NUR provide insight that NUR, a factor that can be modified through intervention, is an important considering in OA.20
A weak, inverse relationship between NUTFBR and body fat percentage corroborates previous research. A higher intake of cereal fibre was related to lower BMI, body fat percentage and percent trunk fat mass, particularly from products containing ≥25% whole grains.31 Higher intake of fibre is linked to lower knee pain intensity26 and better mobility in older adults with OA.21 Potential mechanisms by which greater fibre intake may yield positive outcomes in knee OA is through management of chronic inflammation and obesity. Data from the Osteoarthritis Initiative and Framingham Offspring Osteoarthritis Study show that32 higher intake of dietary fibre may lead to lower body mass longitudinally in symptomatic knee OA; a relationship potentially mediated by C-reactive protein (a pro-inflammatory marker).32 Nonetheless, it is important to recognise that, in the current analysis, the low magnitude of the beta coefficients and wide CI bands emphasise that fibre intake is likely a weak influence on body composition, particularly in comparison to physical activity.
Physical activity and body composition in OA
In this OA subsample from the CLSA data set, physical activity level was associated with body composition. As hypothesised, higher physical activity as self-reported on the PASE, was related to higher lean mass, lower fat mass and lower body fat percentage. After accounting for covariates, for every 100 point increase in the PASE (range 0–793 points), the total body lean mass increased by 1 kg.
People with OA engage in less physical activity than their counterparts.33 A decline in physical activity is likely linked to loss of muscle mass.34 This sarcopenia is related to mortality and impaired function.34 Among nursing home residents (n=122, age ≥70 years), sarcopenia was inversely related to physical activity participation and nutritional status.35 Among obese men and women seeking treatment for weight loss (n=111), sarcopenic obesity was associated with both a lower daily step count (p=0.008) and higher prevalence of sedentary lifestyle (classified as <5000 steps per day) (p=0.017) compared with those without sarcopenic obesity.36 Replacing an hour of sedentary time per day with light activity may offer a strategy to mitigate functional decline, particularly for adults with painful joints.37
Body composition versus total body mass in OA
While physical activity, NUR and fibre intake were associated with elements of body composition, these independent variables were not associated with total mass. The relative ratio of fat free to fat mass may be more important than total body mass in OA. Given that diet interventions have demonstrated a concurrent reduction in both lean and fat mass,13 14 the current findings raise the importance of evaluating body composition, rather than solely mass, when determining the efficacy of diet and physical activity interventions in OA. Among older adults with hip and/or knee OA and normal BMI, lower lean mass was associated with greater pain and poorer physical function.16 Reducing fat mass is also associated with function and performance in OA. Intensive aquatic resistance exercise for OA reduced fat mass and increased walking speed after the 4-month intervention period.38 In fact, a dose-response association was noted, where higher physical activity, as recorded using a daily physical activity diary, was associated with a greater reduction in fat mass.38
Limitations
First, the Short Diet Questionnaire to assess food intake was brief, and the individual items of a NUTHC and NUTFBR were used to categorise the quality of diet. This study did not consider the effects of other food items, distribution of macronutrients or caloric intake.39 Second, questionnaires are subject to bias, including the tools used to measure food intake, NUR and physical activity. This bias reflects both recall (response reflects inability to accurately report a past event) and social desirability (response reflects a desire to seek social approval). Social desirability bias is likely to skew data toward self-reporting better nutrition and physical activity. Nonetheless, the NUR and physical activity tools have been validated, and the physical activity tool requests recall over a relatively short duration, 7 days. Importantly, the use of self-report tools also reflects practicality when collecting data in a large scale study such as the CLSA. Third, environmental conditions, namely outdoor temperature, affect physical activity in knee OA but were not considered in this analysis.40 Similarly, occupational tasks across the life-course could influence body composition outcomes in OA, but this history was not considered. Fourth, CLSA documentation identifies that, at the developmental stage of the CLSA, it was deemed inappropriate to combine dissimilar ethnic groups, while also logistically challenging to obtain a sufficient sample in distinct groups.22 As a result, this data set does not result in a nationally representative sample of the Canadian population.22 Finally, independent variables that yielded small effects on the predicted outcomes may have achieved statistical significance due to the large sample size included in this analysis.
Conclusions
Lifestyle behaviours, characterised by NUR and physical activity, appear more important than dietary intake (eg, fibre or NUTHC) for body composition in older Canadians with OA. This study suggests that body composition may provide more insight than crude measures of body size in understanding OA pathology; and that OA interventions should target both physical activity and the behaviours surrounding nutrition. Future interventions should explore a combination of physical activity and mitigation of NUR through removal of barriers to improved food intake and education on diet quality to improve body composition and outcomes of those living with OA.
Data availability statement
Data may be obtained from a third party and are not publicly available. This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the CLSA is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation. This research has been conducted using the CLSA data set, Baseline Comprehensive Dataset V.3.1, under Application Number 161014. The CLSA is led by Drs Parminder Raina, Christina Wolfson and Susan Kirkland.
Ethics statements
Patient consent for publication
Ethics approval
Ethical approval was received from the Hamilton Integrated Research Ethics Board (Hamilton, Ontario, Canada).
Acknowledgments
The authors would like to acknowledge Professor Paul Stratford from the School of Rehabilitation Science at McMaster University for statistical support.
References
Footnotes
Contributors All authors contributed to the design, analysis and interpretation of data, drafting and final approval of the article. MRM is the guarantor.
Funding JNC-H was supported through a Canadian Institutes of Health Research (CIHR) fellowship award. HK is the Schlegel-University of Waterloo Research Institute for Aging, Research Chair, Nutrition & Aging. MRM is supported by The Arthritis Society Stars Career Development Award, funded by the Canadian Institutes of Health Research-Institute of Musculoskeletal Health and Arthritis. Operating costs were supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (353715 MRM). This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation. This research has been conducted using the CLSA data set, Baseline Comprehensive Dataset V.3.1, under Application Number 161014. The CLSA is led by Drs Parminder Raina, Christina Wolfson and Susan Kirkland.
Disclaimer The opinions expressed in this manuscript are the author's own and do not reflect the views of the Canadian Longitudinal Study on Aging.
Competing interests No, there are no competing interests.
Provenance and peer review Not commissioned; externally peer reviewed by Emmanuel Baah, University of North Carolina System, USA.