Article Text
Abstract
Introduction Gestational diabetes mellitus (GDM) is rapidly increasing worldwide. Globally, 18.4 million pregnancies are complicated by GDM. Despite its known effect, GDM screening is not part of routine antenatal services in Tanzania. There is paucity of data on the magnitude and risk factors for GDM. Therefore, this study sought to determine prevalence and predictors of GDM among pregnant women in Dodoma region, Tanzania from March to August 2018.
Research design and methods A cross-sectional study was carried out in Dodoma region, Tanzania between April and August of 2018. A total of 582 pregnant women were recruited from four local health facilities, where purposive sampling procedure was used to select the region, districts and health facilities. Simple random sampling was used to select study participants. Screening and diagnosis of GDM were performed using the 2013 WHO criteria. Descriptive and inferential analyses were performed using SPSS V.23 to determine prevalence and independent predictors of GDM.
Results Among 582 participants, 160 (27.5%) participants were diagnosed with GDM. GDM was more prevalent in urban areas than rural areas, among overweight participants, among participants with a history of a large for gestational age baby, among participants with a history of caesarean section, and among participants with college or university education. Multiple logistic regression analysis showed that maternal age above 35 years (adjusted OR (AOR) 3.115 (95% CI: 1.165 to 8.359)), pre-eclampsia (AOR 3.684 (95% CI: 1.202 to 5.293)), low physical activity level (AOR 4.758 (95% CI: 2.232 to 10.143)), lack of awareness of GDM (AOR 6.371 (95% CI: 1.944 to 13.919)), alcohol use (AOR 4.477 (95% CI: 1.642 to 12.202)) and family history of diabetes (AOR 2.344 (95% CI: 1.239 to 4.434)) were significantly associated with GDM.
Conclusions Prevalence of GDM is relatively high in Dodoma region. Most pregnant women are unaware of the condition such that it leads to a high-risk lifestyle. Besides, GDM significantly contributes to the number of high-risk pregnancies that go undetected and suboptimally managed. The antenatal care centres offer an optimum platform for screening, preventing and treating GDM by prioritising high-risk women.
- gestational diabetes
- diabetes mellitus
Data availability statement
Data are not available because they contain other unpublished data for future publication.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What this paper adds
It is noted that gestational diabetes mellitus (GDM) has been explored by researchers in Tanzania, mainly, on prevalence and associated factors for GDM. Most of such studies employed former diagnostic criteria as recommended by the WHO.
This study employed the newly recommended WHO approach for GDM diagnosis that helps to highlight the burden and associated predictors as screening for GDM is not a routine practice in Tanzania.
Results from this study reveal data on prevalence and predictors of GDM in Dodoma, Tanzania using the recommended WHO 2013 guidelines where no study of its kind has been conducted to include both rural and urban population groups in Tanzania. Results can be used by all stakeholders as evidence, especially in recommending screening for pregnant women in Tanzania.
Introduction
Gestational diabetes mellitus (GDM) is defined as ‘any degree of glucose intolerance with onset or first recognised during pregnancy’.1 It is associated with both maternal and fetal outcomes.2 3 Globally, 21.3 million pregnancies are associated with hyperglycaemia and out of such status, 18.4 million pregnancies are attributed to GDM.4 A cross-sectional study done in Qatar found the prevalence of GDM to be 6.4%.5 In India, an increase in prevalence from 1% in 1998 to 16% in 2004 was noted.6 Systematic reviews focused on sub-Saharan Africa from 1999 to 2011 found prevalence of GDM to be 9.2% in Ethiopia, both in rural and urban areas, and 3.7% in rural areas only. In South Africa, the prevalence was 3.8% in urban areas and 1.5% in rural areas.6 Also, a recent study conducted in rural Tanzania reported the highest prevalence of GDM of about 39%.7
The prevalence of GDM was found to be high in women with advanced age, body mass index (BMI) of ≥30 kg/m2, family history of diabetes and inactive lifestyle.5 8–12 Up to 10.4% of women between 20 and 49 years old in Africa have hyperglycaemia in pregnancy.4
The pathophysiology of GDM is based on pregnancy because it triggers changes in maternal metabolism to accommodate growth of the fetus. The need for continuous nourishment of the fetus is made possible by complex interactions of the fetoplacental maternal unit, through pregnancy hormones such as growth hormone, corticotrophin-releasing hormone, placental lactogen, progesterone and prolactin. These hormones create insulin resistance to make glucose available for the fetus. Studies have reported additional factors that play a role in the development of GDM including prepregnancy insulin resistance due to increased maternal adipose deposition, decreased exercise and increased caloric intake.3 13 14 Other factors contributing to the development of GDM include family or prepregnancy history of diabetes, age above 25 years, high BMI, multiparity and excessive pregnancy maternal weight gain.15 16
Complications associated with GDM include risk of caesarean section (CS), pre-eclampsia, third to fourth degree perineal tear and subsequent development of type 2 diabetes mellitus (T2DM).17 18 Uncontrolled GDM has been associated with an increased risk of malformations, spontaneous abortion, fetal macrosomia, birth injuries, neonatal hypoglycaemia, polycythaemia and hyperbilirubinaemia.2 3 Long-term outcomes of children with in utero exposure to maternal hyperglycaemia may include higher risks for obesity and T2DM later in life.3
GDM is typically asymptomatic and incidentally, it is identified during screening. Currently, there is no consensus about the screening method and strategies for GDM. Different methods are used in different countries for diagnosis, depending on a country’s resources and hence, results vary such that they are difficult to compare. The methods include fasting blood glucose (FBG) and/or oral glucose tolerance test (OGTT), glucose challenge test and rapid glucose test.19 Fasting and/or OGTT with 75 g of glucose remains the international standard method, as recommended in the updated WHO guidelines.1 Ample evidence supports the importance of screening for GDM in asymptomatic women and controlling glucose levels to improve maternal as well as fetal outcomes.15 20 21 GDM is a treatable condition, mainly by lifestyle modifications. Reduction of animal protein (such as red meat) and higher intake of proteins such as nuts as well as moderate physical activity have been inversely associated with GDM.12 22
In Tanzania, screening and treatment of GDM are not part of the routine package for antenatal services. Such practice has resulted in little knowledge regarding its prevalence in the country. Moreover, data are scarcely reported in some regions. For example, in the Dar es Salaam and Morogoro regions, the prevalence of GDM was 8.5% and 19.5% in Kilimanjaro region.10 23 Results from such studies demonstrate a possible increase in GDM prevalence in Tanzania even though they only included pregnant women of certain gestational ages (GAs). In due regard, these limitations likely mask the true prevalence of GDM in Tanzania. Clear data regarding prevalence and predictors of GDM among pregnant women at any GA are needed to inform policy change and devise effective interventions for prevention, screening as well as treatment. The purpose of this study was to assess prevalence and predictors of GDM among pregnant women in Dodoma region, Tanzania.
Research methods and designs
Study area
This study was carried out in Dodoma region, which lies at 4°–7° latitude south and 35°–37° longitude east. The region has an approximate population of 2 million people with six rural districts and one urban district, namely, Bahi, Chemba, Chamwino, Kondoa, Mpwapwa, Kongwa and Dodoma Urban. There are no routine services focused on preventing and treating GDM. Both urban and rural areas of Dodoma region were studied. In the urban setting, the study was conducted at Chamwino Dispensary and Makole Health Centre, while in the rural setting, the study was carried out at Bahi and Chamwino Health Centres. The participating facilities can serve a total of 1632 women in the antenatal care (ANC) clinic per month (Chamwino 200 and Makole 820, Bahi 460 and Chamwino 152 pregnant women in ANC) covering 80% of all women who attend ANC in Dodoma region.
Study design
A cross-sectional study was carried out that involved screening for GDM among pregnant women at all GAs who attended ANC clinic during the study period.
Study population
The sample included all consenting pregnant women attending ANC in Dodoma region during the study period with no history of DM. They attended ANC in a fasting state for 8 hours since the last meal (supper) or who agreed to attend on the following day in a fasting state. Pregnant women who failed to show up in a fasting state and those who refused to attend on the following day in a fasting state, all pregnant women who were by then taking medications that may interfere with glucose results (for example, quinine anti-malarial) and all pregnant women in ANC with current illness (such as women with high fever) were excluded from taking part in the study.
Sample size estimation
A total of 600 pregnant women who attended ANC in Dodoma region were included in the study. Proportionate size sampling according to the health facilities was done using the formula below:
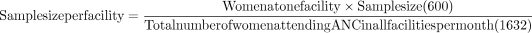
Hence, for Chamwino Dispensary, there were 74 pregnant women, 302 pregnant women at Makole Health Centre, 168 pregnant women at Bahi Health Centre and 56 pregnant women at Chamwino Health Centre. All were purposively selected during the study period to meet the desired sample size of 600.
Sampling techniques
Purposive sampling procedure was used to select the region, districts and health facilities. The aim was to get representation from both rural and urban areas. Out of the seven districts of Dodoma region, Bahi, Chamwino and Dodoma Urban districts were selected. From the selected districts, four health facilities were selected, including Bahi Health Centre and Chamwino Health Centre (rural area); Makole Health Centre (urban area) and Chamwino Dispensary (urban area).
Moreover, simple random sampling procedure was used to select women in a fasting state or who agreed to show up on the following day in a fasting state by using a list of clients who attended ANC. Names were written on pieces of paper and placed in a box with each piece of paper containing only one name. Then, the box was shaken and names were drawn from the box in every facility until the sample size was met.
Data collection methods and tools
Observation and questionnaires were used as data collection methods. The tools involved were a mix of open-ended and closed-ended questions and an observation checklist. The questionnaire had the following eight sections: demographics, socioeconomic status, pregnancy history, current pregnancy, family history, awareness of GDM, lifestyle and physical activity. The observation checklist was used for anthropometric data measurement and investigations.
Questionnaire
Group counselling and an introduction to the study were conducted. The questionnaire was used to collect independent variables. The questionnaire included 6 questions about demographic data, 11 questions sought to assess socioeconomic status (adopted and modified from Tanzania Demographic Health Survey questionnaire), 6 questions were on previous as well as present obstetric data, 10 questions about lifestyle together with family history of diabetes, 8 questions about participants’ awareness of GDM and global physical activity (standardised tool adapted from Global Physical Activity Questionnaire).
Physical measurements/anthropometric measurements
Anthropometric information was collected using the observation checklist. Middle upper arm circumference (MUAC) was obtained by using UNICEF measurement standards and such information was used to categorise women as normal before pregnancy (MUAC <28 cm) and overweight (MUAC ≥28 cm) as it is a proxy for BMI measurement during pregnancy.24 Blood pressure was measured on the left arm once using a manual machine. Women were instructed to clean their perineum and midstream urine was collected in a sterile container to check protein in urine by using dipstick test. Participants were diagnosed with pre-eclampsia if the blood pressure (BP) was ≥140 mm Hg for systolic and diastolic BP ≥90 mm Hg, and protein in urine was ≥+3[(3 g)1].
Biochemical measurements/assessments
Haemoglobin level
Haemoglobin (Hb) levels were measured by finger stick using HemoCue Hb 201+Hb photometer. Participants were classified as non-anaemic (≥110 g/L), mild (95–109 g/L), moderate) (80–94 g/L), severe (65–79 g/L) or life-threatening (<65 g/L) anaemia according to the WHO standard criteria.25
FBG level and OGTT
Screening for GDM was performed by a registered nurse or a request to return the following day was made in case the participant did not attend in a fasting state. FBG was taken from capillary blood and an OGTT with 75 g oral glucose in 300 mL of water was administered orally to participants. After 2 hours, capillary blood glucose was measured again. Conversion of capillary blood glucose level was completed per equation by Bhavadharini et al.26 All participants completed FBG test where fasting was defined as a period of not eating anything for 8 hours, especially from the last meal at night up to the next morning. Thereafter, all clients underwent 2 hours’ OGTT. Diagnosis of GDM was made by using the WHO criteria (WHO 2013). Positive results of GDM were identified on participants’ ANC cards and they were reported to clinicians for proper management as well as follow-up.
Recruitment
Four research assistants (nurses) were recruited and trained for 1 day. They were trained on the objective of the study, interviewing techniques, data collection tools and investigation procedures. Such measures ensured understanding of the tools and for them to observe consistent data collection. Every centre had two trained research assistants (nurses) in order to minimise measurement bias in anthropometric measurements, data collection and diagnosis of GDM.
Data analysis
Data analysis was performed using SPSS V.23. The collected data were entered into the computer and checked for cleanness as well as missing data. Descriptive analysis was performed to understand distribution of participants by demographic characteristics, estimate prevalence and describe patterns of GDM. Component factor analysis was employed for data reduction purposes to establish the weight each item had and to allow for inclusion in the analysis when computing the final composite variable in constructing individual scores for economic status and awareness of participants on GDM. To test the relationship between the independent variables and the categorical dependent variable (GDM status), Χ2 test and multiple logistic regression analysis were used. A 95% CI with a 5% margin of error (0.05) was used as the statistical measure of significance (<0.05 was regarded as significant).
Validity and reliability
This study used a sample size of 582 pregnant mothers to represent the population of Dodoma, which was enough to estimate prevalence of GDM in Dodoma region. Pretesting of the tools (questionnaire and observation checklist) was performed in Kongwa District (Dodoma region) to ensure such tools had to capture the intended information. They were modified for clarity before the study commenced. The questionnaire was reviewed by supervising content experts to check for clarity and consistency.
Results
Participants’ sociodemographic and clinical characteristics
Four participants had incomplete information making a 99.3% response rate. Out of the remaining 596 participants, 14 were found to have diabetes in pregnancy and they were excluded in the analysis. At last, a total of 582 participants with complete questionnaires and results for FBG and/or OGTT were included in the analysis.
Table 1 shows distribution of study participants by sociodemographic and clinical characteristics. The mean age of the participants was 26 years (SD=5.8). Most were from urban areas (63%), 85.7% were married, had primary education (57.6 %, n=335) and they were self-employed (71.5%, n=416). The mean number of pregnancies reported by a participant was 3, the minimum was 1 and maximum 8, and the mean GA for the current pregnancy was 25 weeks, where the minimum and maximum GAs were 12 and 38 weeks, respectively. Anaemia was prevalent at 72.2%, with up to 4.3% of the participants having severe anaemia (Hb), while the mean Hb was 10 g/dL and the minimum as well as the maximum Hb were 7 and 16.6, respectively.
Participants’ sociodemographic and clinical characteristics (N=582)
Among the study participants, 10 (1.7%) were HIV positive; all of them were from urban with their age category between 20 and 34 years; GA was between 20 and 27 weeks and only 2 (20%) were overweight while the remaining had normal BMI (table 1).
Prevalence of GDM
Furthermore, results showed that out of the 582 participants, 160 were diagnosed with GDM, equivalent to a 27.5% prevalence (table 2). Table 2 shows the distribution of participants by the prevalence of GDM and residence type. Proportionally, there was higher prevalence of GDM among participants from urban areas (31.6%) than those residing from rural areas (table 2).
Participants’ distribution by GDM prevalence and residence type
Table 3 shows mean glucose value for FBG test and OGTT results. All participants were subjected to both FBG test and OGTT to diagnose GDM in this study. The FBG test was able to detect up to 96.2% (154) out of the 160 participants who tested positive for GDM. The mean glucose level for FBG was 6.1 mmol/L and the mean glucose level for OGTT was 8.9 mmol/L as illustrated in table 3.
FBG and OGTT mean blood glucose values among participants (N=582)
Table 4 shows the distribution of participants by potential risk factors for GDM in Dodoma. Family history of DM was reported by 8.4%, and 3% had pre-eclampsia on the day of testing (table 4). History of delivery of a baby weighing over 4 kg was reported by 20.6%. The recommended moderate exercise for more than 150 min per week was reported by only 18% of participants (table 4). Prepregnancy overweight and/or obesity (MUAC of >28 cm) was found in 43% of the participants (table 4). Alcohol intake of at least one bottle per day (350 mL) was reported by 3.6% of the participants (table 4). Only 23 of the 582 participants were aware about GDM, equivalent to 4% (urban 18 (78.3%) participants and 5 (21.7%) rural participants) of all study participants, leaving behind the large population of 96% of all participants being unaware of GDM (table 4). Among the participants, 13.2% had a history of abortion, while 3.4% had a history of preterm delivery (table 4).
Distribution of participants by potential risk factors for GDM in Dodoma (N=582)
Predictors of GDM among pregnant women in Dodoma region
Table 5 shows Χ2 test results for factors related with prevalence of GDM among pregnant women in Dodoma region. The GDM was significantly related to participants’ age, residence, education level, history of CS, economic status, pre-eclampsia, physical activity level, MUAC, awareness of GDM, alcohol use, family member with diabetes, family history of diabetes, head of the family and history of large for gestational age (LGA) infant.
Χ2 test results for factors related with prevalence of GDM in Dodoma region (N= 582)
Factors such as age at first conception, occupation, Hb level, history of abortion and number of dependents were analysed but did not show any relationship with GDM (online supplemental file 2).
Supplemental material
Table 6 shows both crude and adjusted OR (AOR) for factors associated with GDM. Findings revealed that advanced maternal age (>35 years), pre-eclampsia, low physical activity level, lack of awareness of GDM, alcohol use and family history of DM are significant independent predictors of GDM, irrespective of known confounders, which were adjusted during the analysis (table 6). Other variables such as MUAC of >28 cm (overweight in pregnancy), high economic status, history of LGA infant and history of CS among participants in a simple logistic regression showed a significant relationship with GDM before adjusting for confounders but no relationship after adjusting for confounders (table 6).
Adjusted OR (AOR) for factors associated with GDM among pregnant women attending ANC at Dodoma region (N=582)
The GDM was found independently associated with age above 35 (AOR 3.115 (95% CI: 1.165 to 8.359)) years, pre-eclampsia (AOR 3.684 (95% CI: 1.202 to 5.293)), low physical activity level (AOR 4.758 (95% CI: 2.232 to 10.143)), lack of awareness of GDM (AOR 6.371 (95% CI: 1.944 to 13.919)), alcohol use (AOR 4.477 (95% CI: 1.642 to 12.202)) and family history of diabetes (AOR 2.344 (95% CI: 1.239 to 4.434)) as shown in table 6.
Factors such as age at first conception, occupation, Hb level, GA, history of abortion and number of dependents were analysed but did not show any relationship with GDM.
Discussion
This study shows prevalence of GDM among pregnant women attending ANC in Dodoma region, Tanzania to be 27.5%. Such prevalence is higher than prevalence reported by other studies conducted in Tanzania whereby it was reported to be 5.9% by Mwanri et al and 19.5% by Njete et al in northern Tanzania.10 23 In the current study, higher prevalence was observed in women with GA above 28 weeks. A study done in Tanzania two decades ago that investigated changes in glucose tolerance in women without diabetes during and after pregnancy found lower FBG and OGTT, indicating no existence of GDM.27 From non-existence of the problem to 27.5% prevalence presented in this study shows increasing trend of the problem and the condition will worsen if no intervention is taken.
The higher prevalence of GDM found in this study could be due to increases in urbanisation, adoption of modern sedentary lifestyles and rise of obesity among women of reproductive age,28 which are reported to be important risk factors for GDM.10 23Another study conducted in Kilimanjaro, Tanzania found a slightly lower GDM prevalence (19.5%) than one found in this study.10 23 Compared with this study, that conducted in Kilimanjaro involved only pregnant women at the GA between 24 and 28 weeks. Maybe prevalence of GDM was underestimated from that study due to the significant number of women left out due to the limitation of GA. Our study did not limit GA of women as per WHO recommendations1 and data collection was done in an ANC where it is free from charges (bills) and any woman can access the service. More than 90% (96.2%) of women with GDM were diagnosed based on FBG. The results are similar to those from the study done in Korongwe, Tanga region in Tanzania where 94.1% of women with GDM were diagnosed based on FBG.7 Also, the study carried out in India by Arora et al showed that FBG diagnosed 94% of women with GDM.29 In low-resource countries, FBG can be used to diagnose GDM as studies show a high ability of FBG to diagnose GDM. Further qualitative studies to explore mothers’ experiences and their views regarding their compliance with fasting requirements of FBG test are needed because this is very critical in establishing whether or not routing GDM screening is possible at ANC.
Our findings differ from the findings in urban Nigeria and Tanzania where the prevalence was 4.8% and 8.4%, respectively.10 30 Again, the lower prevalence of GDM found in other studies might be due to the different diagnostic criteria used. The previous study in Tanzania used the former diagnostic criteria of WHO (WHO 1999) and the study done in Nigeria used the National Diabetes Data group criteria, compared with the current study that used the WHO recommended diagnostic criteria, which has a lower cut-off point than the former recommendation (WHO 2013).
In this study, advanced maternal age (≥35 years) was found to be a significant predictor for GDM. This finding has been widely reported in other studies conducted in Nepal8 and Nigeria.30 Studies conducted in Qatar and Iran, respectively, found that maternal age above 30 years was associated with GDM compared with young age.5 31 Such association may be due to the fact that advanced maternal age is associated with higher parity,8 obesity28 and increased insulin resistance due to parity.
There has been a global decline in the age of pre-diabetes and diabetes onset plus an overall increase in childbearing age. This puts older women of reproductive ages at higher risk for developing GDM3 than women of lower reproductive age. Advanced maternal age has been associated with poor obstetrical outcomes including higher risk for CS32 as well as a primary predictor for GDM, and should be considered when providing care for reproductive-aged women with advanced age.
Family history (first-degree relative) of DM was found in this study to be a significant predictor for GDM. Genetic receptors such as B3-adrenergic genes that can be inherited form one generation to another have been proposed to be responsible for weight gain and insulin resistance33 and hence, higher risk of GDM later in life. Results from this study support those from the study done in Qatar where they found family history, especially paternal history of diabetes, to be significantly associated with GDM.5 Furthermore, a recent study conducted in Dar es Salaam, Tanzania found family history to be one of three statistically significant risk factors for development of GDM.11 A study that was carried out in Canada that assessed GDM’s genetic effects regarding metabolic disease in newborns found that GDM has epigenetic effects on genes that affect the metabolic diseases pathway. This impacts on fetal growth and development, as evidenced by DNA methylation involved in fetal metabolic programming.34 Although the current study did not explore the genetic factor, the observational findings showed that family history of DM is one of the most important predictors of GDM such that it can be used to classify women at high risk for GDM. Such a strategy could be used to identify high-risk women for screening if universal screening is not unfeasible due to resource constraints.
Also, this study found that pre-eclampsia was a predictor of GDM. This could be due to the fact that pre-eclampsia and GDM pathology relate by altering carbohydrate metabolism resulting in vascular-like arteriosclerosis and glomerular filtration dysfunction thereby predisposing an individual to pre-eclampsia.35 This finding correlates with findings in the study conducted to distinguish DNA methylomes present in the human placenta of women with pre-eclampsia and GDM, whereby it was revealed that DNA methylation patterns in human placentas with the stated condition are similar and play a significant role in development of pre-eclampsia including GDM.35 Results from this study support those from the study conducted by an Australian to rule out the effect of GDM treatment on pregnancy outcomes where they found that treatment of GDM reduced the rate of pre-eclampsia by 30%.36 This suggests that pre-eclampsia and GDM are comorbidities that need attention.
Low physical activity among pregnant women was found in this study to be a significant predictor for GDM compared with women with a moderate physical activity level. This finding supports those from a Finnish study conducted by Koivusalo et al where they reported a 30% decreased risk of GDM with such an intervention.12 Another study conducted in China found similar results where women with casual and shorter walking pace were associated with higher risk of GDM than women with usual and longer walking pace.37 A meta-analysis has shown that prepregnancy physical activity in the highest quintile of activity confers a 55% reduction in risk of GDM compared with women in the lowest activity, and early pregnancy physical activity was associated with a statistically significant 25% lower risk of GDM to women in high level of physical activity.38 Low physical activity is a risk factor for GDM due to increased insulin resistance. It is well known that physical activity increases carbohydrates and lipid metabolism by stimulating tissue insulin sensitivity post-exercise. As a result, it is a foundational treatment and prevention strategy for risk reduction.39
Alcohol consumption was a significant predictor for GDM. Women who drank at least one bottle of alcohol every day were at higher risk of GDM than those who stopped after conception and those who do not drink at all. High prevalence of alcohol use among pregnant women (15.1% of 365 pregnant women) was reported by the study done in Dodoma, Tanzania by Mpelo et al using the WHO Alcohol use Disorders Identification Test.40 Our findings are contrary to findings from studies conducted in Iran41 and Congo.42 The study in Iran involved a very small sample size, which might have caused bias in the study. The study in Congo used national recommendation diagnostic criteria and not the WHO (WHO 2013) diagnostic criteria.
Low awareness about the disease was found to be a significant predictor for GDM. According to our study, women who lacked awareness were six times more likely to have GDM than those who had awareness. Awareness of a disease is highly related to implementation of healthy behaviour on preventing disease and its complication.43–45 This is supported by the study done on health literacy in occurrence and management of the disease where individuals with lower level of health literacy are prone to inadequate use of health preventive measures, increase incidence of chronic illness, greater healthcare utilisation and poor disease outcome compared with those with the said literacy.44 46 47 Studies have shown other countries like India and United Arab Emirates where about 75% and 73.5% of study participants, respectively, had awareness of GDM.48 49 Generally, there was low awareness of GDM, irrespective of type of residence. Results highlight the need for awareness campaigns to reach women both in rural and urban areas.
Prepregnancy obesity (MUAC ≥28 cm) has been widely reported as an important predictor for GDM10 23 whereby in this study, it was shown as a significant predictor for GDM when not adjusted with other confounders and a borderline relationship (p=0.072) with GDM when all confounders are adjusted. Clinically, prepregnancy obesity is an important predictor for GDM, as body fat percentage has a proportional relationship with insulin resistance13 although in this study it was shown to be statistically not significant. This tells us that prepregnancy overweight is an important determinant of GDM and for successful prevention of GDM, interventions should start from the prepregnancy period.
From this study, higher economic status, urban residence and advanced education level (secondary and university education) were found to be significant predictors of GDM when not adjusted with confounders and not significant predictors when confounders where adjusted. This is alarming given that GDM is not a matter of being educated or living in urban areas because even those educated participants are at risk of GDM. Hence, an emphasis on awareness and lifestyle modifications can help to combat GDM.
This study did not follow up women with positive GDM to explore the pregnancy outcome and plasma glucose level during delivery and postpartum period. Future studies should consider cohort design where women exposed to GDM can be prospectively followed to determine its association with the pregnancy outcomes. In addition, antiretroviral (ARV) use is known to affect glucose metabolism, resulting to hyperglycaemia, which is not necessarily GDM. These may lead to misdiagnosing GDM. In our study, participants who were HIV positive, their ARV status was not explored. That may have biased our results. However, there were only four clients with HIV who were GDM positive. That did not significantly affect the prevalence of GDM reported in our study.
Conclusion
Prevalence of GDM in Tanzania is rapidly increasing. The strongest risk factors in this study were individual, family and lifestyle factors. They include advanced maternal age, family history of diabetes, low physical activity level and alcohol use. Based on the 2013 diagnostic criteria for GDM, close to one-third of pregnancies in our setting were affected by GDM. There are important maternal and fetal considerations for pregnancies that are complicated by GDM. This represents a significant number of high-risk pregnancies that are currently being undetected and suboptimally managed in our setting.
Based on these findings, to achieve optimal pregnancy outcomes and prevent long-term complications of GDM, routine screening of pregnant women should be adopted and incorporated in ANC services for early detection as well as timely initiation of treatment. An effective diagnostic method should be used with clear links to evidence-based treatment and follow-up. Due to resource constraints, high-risk women could be identified and prioritised for screening. Findings highlight the urgent need for community-level awareness of GDM among women of reproductive age. Promotion of healthy lifestyles should be emphasised as part of interventions to prevent and treat GDM, obesity and other non-communicable diseases.
In addition, preconception care should be promoted, especially components of risk assessment to help women assess health risks before conception and receive treatment. Further longitudinal interventional studies should be conducted, following women with GDM until delivery and puerperium so as to establish treatment options for better pregnancy outcomes. Additional studies should follow up women with positive GDM to explore the pregnancy outcome and plasma glucose level during delivery and postpartum period.
Supplemental material
Data availability statement
Data are not available because they contain other unpublished data for future publication.
Ethics statements
Ethics approval
Permission to conduct the study was obtained from the Ethical Clearance Committee of the University of Dodoma (UDOM/DRP/134/VOL V/23-22) (see online supplemental file 1) and permission to carry out the study was obtained from the regional medical officer, district executive director and nursing in-charge (matron) of the four participating health facilities. Both verbal and written informed consent were given to each participant before taking part in the study. The purpose of the study was explained to women with careful review of risks and benefits from their participation. All procedures involved in the data collection process were communicated to the women before data collection. For those who agreed, they were included in the study until the sample size for all facilities was achieved.
Acknowledgments
The authors would like to recognise the contribution of the regional medical officers and district medical officers of Bahi, Chamwino and Dodoma Urban districts for their support, all nurses and doctors working in the antenatal clinics at Makole Hospital, Bahi and Chamwino Health Centres and Chamwino Dispensary.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors This work is a result of a collaboration of all authors as follows: MBM contributed to the conceptualisation, methodology, data collection, data analysis, writing the first draft of the manuscript and review. SMK supervised the study and wrote the methodology, and performed data analysis and manuscript review. MJM supervised the study and participated in writing the literature review, methodology, analysis of data and manuscript review. AIE participated in conceptualising and writing the methodology (screening for GDM), results writing and manuscript review. All authors read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests There are no competing interests.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.


